Human Papillomavirus Vaccine Effectiveness and Herd Protection in Young Women
- PMID: 30670582
- PMCID: PMC6361347
- DOI: 10.1542/peds.2018-1902
Human Papillomavirus Vaccine Effectiveness and Herd Protection in Young Women
Abstract
Background: Clinical trials of the 4-valent human papillomavirus (HPV) vaccine demonstrate high efficacy, but surveillance studies are essential to examine the long-term impact of vaccine introduction on HPV prevalence in community settings. The aims of this study were to determine during the 11 years after vaccine introduction the prevalence of (1) vaccine-type HPV in adolescent and young adult women who were vaccinated (to assess vaccine effectiveness) and (2) vaccine-type HPV in women who were unvaccinated (to assess herd protection).
Methods: Young women 13 to 26 years of age were recruited from hospital-based and community health clinics for 4 surveillance studies from 2006 to 2017. We determined the proportion of vaccinated and unvaccinated women who were positive for vaccine-type HPV across the studies, and the odds of positivity for vaccine-type HPV using logistic regression; all analyses were propensity score-adjusted to control for between-wave differences in participant characteristics.
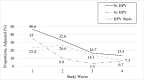
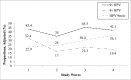
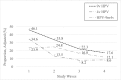
Results: Vaccination rates increased from 0% to 84.3% (97% of study participants received the 4-valent vaccine). Among women who were vaccinated, 4-valent vaccine-type HPV detection decreased from 35% to 6.7% (80.9% decline; odds ratio 0.13, 95% confidence interval 0.08 to 0.22). Among women who were unvaccinated, 4-valent vaccine-type HPV detection decreased from 32.4% to 19.4% (40% decline; odds ratio 0.50, 95% confidence interval 0.26 to 0.97). Estimated vaccine effectiveness was 90.6% in wave 3 and 80.1% in wave 4.
Conclusions: In this study in which trends in HPV in a US community >10 years after 4-valent HPV vaccine introduction and after 9-valent vaccine introduction were examined, we found evidence of vaccine effectiveness and herd protection. Further research is needed to examine trends in 9-valent vaccine-type HPV after higher rates of vaccination are achieved.
Copyright © 2019 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Brown has received an investigator initiated studies program award from Merck entitled “Cervical Cancer Prevention in Kenya” and owns stock in shares of Merck & Co, Inc. Dr Franco has occasionally served as consultant to companies involved with human papillomavirus (HPV) diagnostics (Roche, BD, and Abbott) and HPV vaccines (Merck and GSK). Dr Kahn served as cochair of a study of HPV vaccines in men with HIV. The study was funded by the National Institutes of Health, but Merck provided vaccine and serology testing; the other authors have indicated they have no potential conflicts of interest to disclose.
Figures
Comment in
-
Three Important Findings From a Study on HPV "Real World" Effectiveness.Pediatrics. 2019 Feb;143(2):e20183427. doi: 10.1542/peds.2018-3427. Epub 2019 Jan 22. Pediatrics. 2019. PMID: 30670583 No abstract available.
References
-
- Bosch FX, de Sanjosé S. Chapter 1: human papillomavirus and cervical cancer–burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;(31):3–13 - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention; Advisory Committee on Immunization Practices . Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1–24 - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, et al. ; Centers for Disease Control and Prevention . Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) [published correction appears in MMWR Recomm Rep. 2014;63(49):1182]. MMWR Recomm Rep. 2014;63(RR-05):1–30 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

