Effects of Friend Virus Infection and Regulatory T Cells on the Antigen Presentation Function of B Cells
- PMID: 30670616
- PMCID: PMC6343038
- DOI: 10.1128/mBio.02578-18
Effects of Friend Virus Infection and Regulatory T Cells on the Antigen Presentation Function of B Cells
Abstract
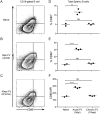
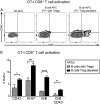
Friend virus (FV) is a naturally occurring mouse retrovirus that infects dividing cells of the hematopoietic lineage, including antigen-presenting cells (APCs). The infection of APCs by viruses often induces their dysfunction, and it has been shown that FV infection reduces the ability of dendritic cells (DCs) to prime critical CD8+ T cell responses. Nonetheless, mice mount vigorous CD8+ T cell responses, so we investigated whether B cells might serve as alternative APCs during FV infection. Direct ex vivo analysis of B cells from FV-infected mice revealed that infected but not uninfected B cells upregulated expression of the costimulatory molecules CD80, CD86, and CD40, as well as major histocompatibility complex class II (MHC-II) molecules. Furthermore, in vitro studies showed that, compared to uninfected B cells from the same mice, the FV-infected B cells had significantly enhanced APC function, as measured by their capacity to prime CD8+ T cell activation and proliferation. Thus, in contrast to DCs, infection of B cells with FV enhanced their APC capacity and ability to stimulate the CD8+ T cell responses essential for virus control. FV infections also induce the activation and expansion of regulatory T cells (Tregs), so it was of interest to determine the impact of Tregs on B cell activation. The upregulation of costimulatory molecule expression and APC function of B cells was even more strongly enhanced by in vivo depletion of regulatory T cells than infection. Thus, Tregs exert potent homeostatic suppression of B cell activation that is partially overcome by FV infection.IMPORTANCE The primary role of B cells in immunity is considered the production of pathogen-specific antibodies, but another, less-well-studied, function of B cells is to present foreign antigens to T cells to stimulate their activation and proliferation. Dendritic cells (DCs) are considered the most important antigen-presenting cells (APCs) for CD8+ T cells, but DCs lose APC function when infected with Friend virus (FV), a model retrovirus of mice. Interestingly, B cells were better able to stimulate CD8+ T cell responses when they were infected with FV. We also found that the activation status of B cells under homeostatic conditions was potently modulated by regulatory T cells. This study illustrates an important link between B cell and T cell responses and illustrates an additional mechanism by which regulatory T cells suppress critical T cell responses during viral infections.
Keywords: B-cell responses; CD8+ T cells; antigen presentation; regulatory T cells; retroviruses.
Figures




Similar articles
-
B Cell Requirement for Robust Regulatory T Cell Responses to Friend Retrovirus Infection.mBio. 2017 Aug 1;8(4):e01122-17. doi: 10.1128/mBio.01122-17. mBio. 2017. PMID: 28765225 Free PMC article.
-
In vitro suppression of CD8+ T cell function by Friend virus-induced regulatory T cells.J Immunol. 2006 Mar 15;176(6):3342-9. doi: 10.4049/jimmunol.176.6.3342. J Immunol. 2006. PMID: 16517701
-
Friend retrovirus infection of myeloid dendritic cells impairs maturation, prolongs contact to naive T cells, and favors expansion of regulatory T cells.Blood. 2007 Dec 1;110(12):3949-58. doi: 10.1182/blood-2007-05-092189. Epub 2007 Aug 15. Blood. 2007. PMID: 17699743
-
Combination of nanoparticle-based therapeutic vaccination and transient ablation of regulatory T cells enhances anti-viral immunity during chronic retroviral infection.Retrovirology. 2016 Apr 14;13:24. doi: 10.1186/s12977-016-0258-9. Retrovirology. 2016. PMID: 27076190 Free PMC article. Review.
-
Host genetic factors that control immune responses to retrovirus infections.Vaccine. 2008 Jun 6;26(24):2981-96. doi: 10.1016/j.vaccine.2008.01.004. Epub 2008 Jan 22. Vaccine. 2008. PMID: 18255203 Review.
Cited by
-
Friend retrovirus studies reveal complex interactions between intrinsic, innate and adaptive immunity.FEMS Microbiol Rev. 2019 Sep 1;43(5):435-456. doi: 10.1093/femsre/fuz012. FEMS Microbiol Rev. 2019. PMID: 31087035 Free PMC article. Review.
-
Influenza virus infection enhances tumour-specific CD8+ T-cell immunity, facilitating tumour control.PLoS Pathog. 2024 Jan 25;20(1):e1011982. doi: 10.1371/journal.ppat.1011982. eCollection 2024 Jan. PLoS Pathog. 2024. PMID: 38271469 Free PMC article.
-
B cell-T cell interplay in immune regulation: A focus on follicular regulatory T and regulatory B cell functions.Front Cell Dev Biol. 2022 Sep 23;10:991840. doi: 10.3389/fcell.2022.991840. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36211467 Free PMC article. Review.
-
Cytotoxic CD4+ T cells in chronic viral infections and cancer.Front Immunol. 2023 Oct 25;14:1271236. doi: 10.3389/fimmu.2023.1271236. eCollection 2023. Front Immunol. 2023. PMID: 37965314 Free PMC article. Review.
-
Antibody-independent functions of B cells during viral infections.PLoS Pathog. 2021 Jul 22;17(7):e1009708. doi: 10.1371/journal.ppat.1009708. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34293057 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

