Phosphoproteomics of Arabidopsis Highly ABA-Induced1 identifies AT-Hook-Like10 phosphorylation required for stress growth regulation
- PMID: 30670655
- PMCID: PMC6369736
- DOI: 10.1073/pnas.1819971116
Phosphoproteomics of Arabidopsis Highly ABA-Induced1 identifies AT-Hook-Like10 phosphorylation required for stress growth regulation
Abstract
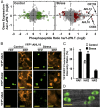
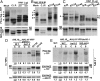
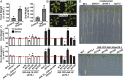
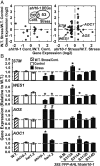
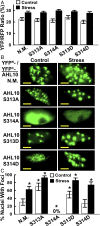
The clade A protein phosphatase 2C Highly ABA-Induced 1 (HAI1) plays an important role in stress signaling, yet little information is available on HAI1-regulated phosphoproteins. Quantitative phosphoproteomics identified phosphopeptides of increased abundance in hai1-2 in unstressed plants and in plants exposed to low-water potential (drought) stress. The identity and localization of the phosphoproteins as well as enrichment of specific phosphorylation motifs indicated that these phosphorylation sites may be regulated directly by HAI1 or by HAI1-regulated kinases including mitogen-activated protein kinases, sucrose non-fermenting-related kinase 2, or casein kinases. One of the phosphosites putatively regulated by HAI1 was S313/S314 of AT-Hook-Like10 (AHL10), a DNA-binding protein of unclear function. HAI1 could directly dephosphorylate AHL10 in vitro, and the level of HAI1 expression affected the abundance of phosphorylated AHL10 in vivo. AHL10 S314 phosphorylation was critical for restriction of plant growth under low-water potential stress and for regulation of jasmonic acid and auxin-related gene expression as well as expression of developmental regulators including Shootmeristemless These genes were also misregulated in hai1-2 AHL10 S314 phosphorylation was required for AHL10 complexes to form foci within the nucleoplasm, suggesting that S314 phosphorylation may control AHL10 association with the nuclear matrix or with other transcriptional regulators. These data identify a set of HAI1-affected phosphorylation sites, show that HAI1-regulated phosphorylation of AHL10 S314 controls AHL10 function and localization, and indicate that HAI1-AHL10 signaling coordinates growth with stress and defense responses.
Keywords: AT-hook; drought; phosphoproteomics; phosphorylation; protein phosphatase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Verslues PE. ABA and cytokinins: Challenge and opportunity for plant stress research. Plant Mol Biol. 2016;91:629–640. - PubMed
-
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–679. - PubMed
-
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signalling. Trends Plant Sci. 2010;15:395–401. - PubMed
-
- de Zelicourt A, Colcombet J, Hirt H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016;21:677–685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

