Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders
- PMID: 30670701
- PMCID: PMC6342993
- DOI: 10.1038/s41467-018-08264-w
Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders
Erratum in
-
Publisher Correction: Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders.Nat Commun. 2019 Feb 8;10(1):734. doi: 10.1038/s41467-019-08716-x. Nat Commun. 2019. PMID: 30737404 Free PMC article.
Abstract
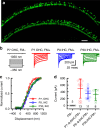
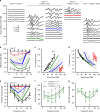
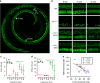
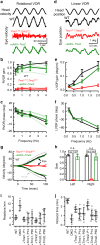
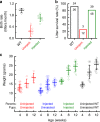
Fifty percent of inner ear disorders are caused by genetic mutations. To develop treatments for genetic inner ear disorders, we designed gene replacement therapies using synthetic adeno-associated viral vectors to deliver the coding sequence for Transmembrane Channel-Like (Tmc) 1 or 2 into sensory hair cells of mice with hearing and balance deficits due to mutations in Tmc1 and closely related Tmc2. Here we report restoration of function in inner and outer hair cells, enhanced hair cell survival, restoration of cochlear and vestibular function, restoration of neural responses in auditory cortex and recovery of behavioral responses to auditory and vestibular stimulation. Secondarily, we find that inner ear Tmc gene therapy restores breeding efficiency, litter survival and normal growth rates in mouse models of genetic inner ear dysfunction. Although challenges remain, the data suggest that Tmc gene therapy may be well suited for further development and perhaps translation to clinical application.
Conflict of interest statement
J.R.H. is a consultant to several companies focused on development of inner ear therapeutics. J.R.H. and G.S.G. hold patents on
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

