Group II Metabotropic Glutamate Receptors Mediate Presynaptic Inhibition of Excitatory Transmission in Pyramidal Neurons of the Human Cerebral Cortex
- PMID: 30670948
- PMCID: PMC6333023
- DOI: 10.3389/fncel.2018.00508
Group II Metabotropic Glutamate Receptors Mediate Presynaptic Inhibition of Excitatory Transmission in Pyramidal Neurons of the Human Cerebral Cortex
Abstract
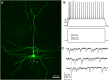
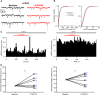
Group II metabotropic glutamate receptor (mGluR) ligands are potential novel drugs for neurological and psychiatric disorders, but little is known about the effects of these compounds at synapses of the human cerebral cortex. Investigating the effects of neuropsychiatric drugs in human brain tissue with preserved synaptic circuits might accelerate the development of more potent and selective pharmacological treatments. We have studied the effects of group II mGluR activation on excitatory synaptic transmission recorded from pyramidal neurons of cortical layers 2-3 in acute slices derived from surgically removed cortical tissue of people with epilepsy or tumors. The application of a selective group II mGluR agonist, LY354740 (0.1-1 μM) inhibited the amplitude and frequency of action potential-dependent spontaneous excitatory postsynaptic currents (sEPSCs). This effect was prevented by the application of a group II/III mGluR antagonist, CPPG (0.1 mM). Furthermore, LY354740 inhibited the frequency, but not the amplitude, of action potential-independent miniature EPSCs (mEPSCs) recorded in pyramidal neurons. Finally, LY354740 did slightly reduce cells' input resistance without altering the holding current of the neurons recorded in voltage clamp at -90 mV. Our results suggest that group II mGluRs are mainly auto-receptors that inhibit the release of glutamate onto pyramidal neurons in layers 2-3 in the human cerebral cortex, thereby regulating network excitability. We have demonstrated the effect of a group II mGluR ligand at human cortical synapses, revealing mechanisms by which these drugs could exert pro-cognitive effects and treat human neuropsychiatric disorders.
Keywords: EPSC; cognitive enhancer; epilepsy; glutamatergic; human cortex; mGluR; presynaptic receptor; transmitter release.
Figures



References
-
- Aronica E., Gorter J. A., Ijlst-Keizers H., Rozemuller A. J., Yankaya B., Leenstra S., et al. (2003). Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur. J. Neurosci. 17 2106–2118. 10.1046/j.1460-9568.2003.02657.x - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

