Characteristics of Seizure and Antiepileptic Drug Utilization in Outpatients With Autoimmune Encephalitis
- PMID: 30671012
- PMCID: PMC6331521
- DOI: 10.3389/fneur.2018.01136
Characteristics of Seizure and Antiepileptic Drug Utilization in Outpatients With Autoimmune Encephalitis
Abstract
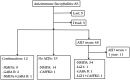
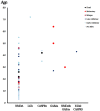
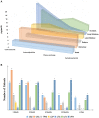
Autoimmune encephalitis (AE) is one kind of encephalitis that associates with specific neuronal antigens. Most patients with AE likely suffer from seizures, but data on the characteristics of seizure and antiepileptic drugs (AEDs) utilization in this patient group remains limited. This study aimed to report the clinical status of seizure and AEDs treatment of patients with AE, and to evaluate the relationship between AEDs discontinuation and seizure outcomes. Patients with acute neurological disorders and anti-N-methyl-D-aspartate receptor (NMDAR), γ-aminobutyric acid B receptor (GABABR), leucine-rich glioma inactivated 1, or contactin-associated protein-like 2 (CASPR2) antibodies were included. As patients withdrew from AEDs, they were divided into the early withdrawal (EW, AEDs used ≤3 months) and late withdrawal (LW, AEDs used >3 months) groups. Seizure remission was defined as having no seizures for at least 1 year after the last time when AEDs were administered. Seizure outcomes were assessed on the basis of remission rate. The factors affecting the outcomes were assessed through Spearman analysis. In total, we enrolled 75 patients (39 patients aged <16 years, male/female = 39/36) for follow-up, which included 67 patients with anti-NMDAR encephalitis, 4 patients with anti-GABABR encephalitis, 2 patients with anti-voltage-gated potassium channel encephalitis, and 2 patients with coexisting antibodies. Among the 34 enrolled patients with anti-NMDAR encephalitis who were withdrawn from AEDs, only 5.8% relapse was reported during the 1-year follow-up, with no significant difference in the percentage of relapse between the EW and LW groups (P = 0.313). Fifteen patients (an average age of 6.8, 14 patients with anti-NMDAR encephalitis and 1 patient with anti-CASPR2 encephalitis) presented seizure remission without any AEDs. Seventy five percent of patients with anti-GABABR antibodies developed refractory seizure. Other risk factors which contributed to refractory seizure and seizure relapse included status epilepticus (P = 0.004) and cortical abnormalities (P = 0.028). Given this retrospective data, patients with AE have a high rate of seizure remission, and the long-term use of AEDs may not be necessary to control the seizure. Moreover, seizures in young patients with anti-NMDAR encephalitis presents self-limited. Patients with anti-GABABR antibody, status epilepticus, and cortical abnormalities are more likely to develop refractory seizure or seizure relapse.
Keywords: antiepileptic drug withdrawal; autoimmune encephalitis; outpatients; refractory seizure; seizure remission.
Figures
References
-
- Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain (2010) 133 (Pt 6):1655–67. 10.1093/brain/awq113 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

