Traceability of Blood Transfusions and Reporting of Adverse Reactions in Developing Countries: A Six-Year Postpilot Phase Experience in Burkina Faso
- PMID: 30671095
- PMCID: PMC6317082
- DOI: 10.1155/2018/7938130
Traceability of Blood Transfusions and Reporting of Adverse Reactions in Developing Countries: A Six-Year Postpilot Phase Experience in Burkina Faso
Abstract
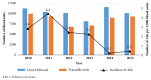
Traceability is an essential tool for haemovigilance and transfusion safety. In Burkina Faso, the implementation of haemovigilance has been achieved as part of a pilot project from 2005 to 2009. Our study aims to evaluate the traceability of blood transfusions and reporting of adverse reactions over the 6-year postpilot phase. A cross-sectional study including all blood units ordered between 2010 and 2015 has been conducted in public and private health care facilities supplied with blood products by the transfusion center of Bobo-Dioulasso. The complete traceability was possible for 83.5% of blood units delivered. Adverse reactions were reported in 107 cases representing 2.1/1,000 blood units per annum. Transfusions of wrong blood to wrong patient were reported in 13 cases. Our study shows that the haemovigilance system in Burkina Faso must be improved. Healthcare workers have to be sensitized on how traceability and haemovigilance could impact the quality of care provided to patients.
Figures


References
-
- Somagari D. R., Sriram C. S., Rachamalla C. K., et al. Haemovigilance study at a tertiary care hospital in the north-east of India. ISBT Science Series. 2015;10(2):61–64. doi: 10.1111/voxs.12182. - DOI
-
- Ministère de la santé et de l'action humanitaire. Loi no 93-5 du 4 janvier 1993 relative à la sécurité en matière de transfusion sanguine et de médicament. Journal officiel français. 1993:237–245.
LinkOut - more resources
Full Text Sources

