Induction of superovulation in mature mice and rats using serum of spayed female dogs
- PMID: 30671107
- PMCID: PMC6333601
- DOI: 10.5625/lar.2018.34.4.211
Induction of superovulation in mature mice and rats using serum of spayed female dogs
Abstract
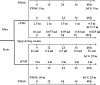
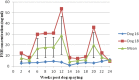
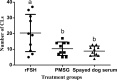
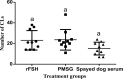
The following experiments were designed to examine the effect of serum of spayed dogs on superovulation response in mice and rats. In Experiment 1, female mice at diestrus (n=30) were divided into three equal groups and superovulated with either administration of 5 IU pregnant mare serum gonadotropin (PMSG) or recombinant follicle stimulating hormone (rFSH) (reducing dose from 2.5 to 0.5 IU) and 5 IU human chorionic gonadotropin (hCG) administered 48h later. Serum of spayed dogs was administered intraperitoneally at a reduced dose from 0.1 to 0.025 mL in a 48 h period. In Experiment 2, female rats (n=30) at diestrus stage were divided into three equal groups. Superovulation was induced using either 30 IU PMSG, or a dose reduced from 5 to 1 IU rFSH and 25 IU hCG administered 48h later. Serum of spayed dogs was administered in a reduced dose from 0.6 to 0.1 mL in a 48 hour period. Female mice and rats were mated 24 h following hCG administration. On day 14 after mating, animals were euthanized and ovarian sections were fixed for histopathological evaluation and corpus luteum (CL) counting. No significant difference observed in mean (±SEM) number of CLs between the PMSG group and the mice that received serum of spayed dog (10.4±1.3 vs 9.2±1.0). Mean (±SEM) number of CLs tended to be lower in rats that received serum of spayed dog than those of rats which received either PMSG or rFSH (15.1±1.9 vs 23.6±3.1 and 23.1±2.9, P=0.06, respectively). In conclusion, serum of spayed dogs is able to induce a superovulatory response in mice and rats.
Keywords: FSH; PMSG; Superovulation; spayed dog serum.
Conflict of interest statement
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
Figures
References
-
- Albers-Wolthers CH, de Gier J, Oei CH, Schaefers-Okkens AC, Kooistra HS. Validation of a noninvasive diagnostic tool to verify neuter status in dogs: The urinary FSH to creatinine ratio. Theriogenology. 2016;86(5):1376–1381. - PubMed
-
- Beijerink NJ, Buijtels JJ, Okkens AC, Kooistra HS, Dieleman SJ. Basal and GnRH-induced secretion of FSH and LH in anestrous versus ovariectomized bitches. Theriogenology. 2007;67(5):1039–1045. - PubMed
-
- Boland MP, Goulding D, Roche JF. Alternative gonadotrophins for superovulation in cattle. Theriogenology. 1991;35(1):5–17.
-
- Hasegawa A, Mochida K, Inoue H, Noda Y, Endo T, Watanabe G, Ogura A. High-Yield Superovulation in Adult Mice by Anti-Inhibin Serum Treatment Combined with Estrous Cycle Synchronization. Biol Reprod. 2016;94(1):21. - PubMed
-
- Hoppen HO. The equine placenta and equine chorionic gonadotrophin--an overview. Exp Clin Endocrinol. 1994;102(3):235–243. - PubMed

