A Novel Non-invasive Technique of Measuring Bilirubin Levels Using BiliCapture
- PMID: 30671181
- PMCID: PMC6330178
- DOI: 10.5001/omj.2019.05
A Novel Non-invasive Technique of Measuring Bilirubin Levels Using BiliCapture
Abstract
Objectives: In preterm infants, hyperbilirubinemia is common and can impair the central nervous system. The tests available for measuring bilirubin is to collect blood from heel pricking and occasionally taking blood samples from inserted cannulas, which is painful. Therefore, there is a need to develop a non-invasive device to detect bilirubin levels in newborns and interpret the severity of jaundice.
Methods: We conducted a cross-sectional study of 100 neonates. Patient data was collected between June 2015 and December 2016 from King Khalid Hospital at Al-Majma'ah, Saudi Arabia, and Alpine Hospital, Gurgaon, India. The mean gestational age of neonates was 39.0 weeks. Total bilirubin was measured using a transcutaneous bilirubinometer on the forehead and obtaining optical imaging through scanning of conjunctiva of eyes, also referred to as BiliChek and BiliCapture, respectively. Later the blood samples were obtained from these patients and tested in the laboratory to determine total serum bilirubin (TSB) levels.
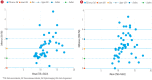
Results: The concentration of bilirubin as measured from serum, BiliChek, and BiliCapture were 10.7±2.0, 11.6±2.7, and 13.1±2.3 mg/dL, respectively. Correlation was high between TSB and BiliChek (r2 = 0.88) and between TSB and BiliCapture (r2 = 0.73). The Bland-Altman plots showed good agreement when comparing bilirubin values for both BiliChek and BiliCapture devices. Bilirubin measurement was further checked for the sensitivity and specificity and was 88.0% and 76.0% using BiliChek and 92.0% and 75.6% using BiliCapture, respectively.
Conclusions: The optical imaging of conjunctiva for bilirubin assay is a safe alternative to a laboratory bilirubin assay and transcutaneous bilirubinometer BiliChek.
Keywords: Bilirubin; Hyperbilirubinemia; Jaundice; Neonate.
Figures




References
LinkOut - more resources
Full Text Sources
