CRISPR/Cas9-mediated deletion of the Wiskott-Aldrich syndrome locus causes actin cytoskeleton disorganization in murine erythroleukemia cells
- PMID: 30671311
- PMCID: PMC6339507
- DOI: 10.7717/peerj.6284
CRISPR/Cas9-mediated deletion of the Wiskott-Aldrich syndrome locus causes actin cytoskeleton disorganization in murine erythroleukemia cells
Abstract
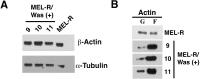
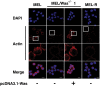
Wiskott-Aldrich syndrome (WAS) is a recessive X-linked inmmunodeficiency caused by loss-of-function mutations in the gene encoding the WAS protein (WASp). WASp plays an important role in the polymerization of the actin cytoskeleton in hematopoietic cells through activation of the Arp2/3 complex. In a previous study, we found that actin cytoskeleton proteins, including WASp, were silenced in murine erythroleukemia cells defective in differentiation. Here, we designed a CRISPR/Cas9 strategy to delete a 9.5-kb genomic region encompassing the Was gene in the X chromosome of murine erythroleukemia (MEL) cells. We show that Was-deficient MEL cells have a poor organization of the actin cytoskeleton that can be recovered by restoring Was expression. We found that whereas the total amount of actin protein was similar between wild-type and Was knockout MEL cells, the latter exhibited an altered ratio of monomeric G-actin to polymeric F-actin. We also demonstrate that Was overexpression can mediate the activation of Bruton's tyrosine kinase. Overall, these findings support the role of WASp as a key regulator of F-actin in erythroid cells.
Keywords: Actin cytoskeleton; Bruton tyrosine kinase; CRISPR/Cas9; Erythroleukemia cells; Wiskott-Aldrich.
Conflict of interest statement
Dora B. Krimer is an Academic Editor for PeerJ.
Figures






References
-
- Albert MH, Bittner TC, Nonoyama S, Notarangelo LD, Burns S, Imai K, Espanol T, Fasth A, Pellier I, Strauss G, Morio T, Gathmann B, Noordzij JG, Fillat C, Hoenig M, Nathrath M, Meindl A, Pagel P, Wintergerst U, Fischer A, Thrasher AJ, Belohradsky BH, Ochs HD. X-linked thrombocytopenia (XLT) due to WAS mutations: clinical characteristics, long-term outcome, and treatment options. Blood. 2010;115:3231–3238. doi: 10.1182/blood-2009-09-239087. - DOI - PubMed
-
- Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, Gonzalez-Aller C, Hiester A, De Boer M, Harbeck RJ, Oyer R, Johnson GL, Roos D. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4654–4659. doi: 10.1073/pnas.080074897. - DOI - PMC - PubMed
-
- Baba Y, Nonoyama S, Matsushita M, Yamadori T, Hashimoto S, Imai K, Arai S, Kunikata T, Kurimoto M, Kurosaki T, Ochs HD, Yata J, Kishimoto T, Tsukada S. Involvement of wiskott-aldrich syndrome protein in B-cell cytoplasmic tyrosine kinase pathway. Blood. 1999;93:2003–2012. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

