Entamoeba histolytica Up-Regulates MicroRNA-643 to Promote Apoptosis by Targeting XIAP in Human Epithelial Colon Cells
- PMID: 30671387
- PMCID: PMC6333105
- DOI: 10.3389/fcimb.2018.00437
Entamoeba histolytica Up-Regulates MicroRNA-643 to Promote Apoptosis by Targeting XIAP in Human Epithelial Colon Cells
Abstract
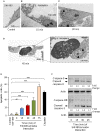
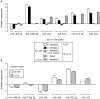
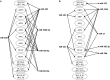
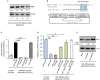
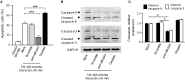
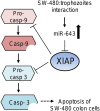
MicroRNAs (miRNAs) are small non-coding RNAs that function as negative regulators of gene expression. Recent evidences suggested that host cells miRNAs are involved in the progression of infectious diseases, but its role in amoebiasis remains largely unknown. Here, we reported an unexplored role for miRNAs of human epithelial colon cells during the apoptosis induced by Entamoeba histolytica. We demonstrated for the first time that SW-480 colon cells change their miRNAs profile in response to parasite exposure. Our data showed that virulent E. histolytica trophozoites induced apoptosis of SW-480 colon cells after 45 min interaction, which was associated to caspases-3 and -9 activation. Comprehensive profiling of 667 miRNAs using Taqman Low-Density Arrays showed that 6 and 15 miRNAs were significantly (FC > 1.5; p < 0.05) modulated in SW-480 cells after 45 and 75 min interaction with parasites, respectively. Remarkably, no significant regulation of the 6-miRNAs signature (miR-526b-5p, miR-150, miR-643, miR-615-5p, miR-525, and miR-409-3p) was found when SW-480 cells were exposed to non-virulent Entamoeba dispar. Moreover, we confirmed that miR-150, miR-643, miR-615-5p, and miR-525 exhibited similar regulation in SW-480 and Caco2 colon cells after 45 min interaction with trophozoites. Exhaustive bioinformatic analysis of the six-miRNAs signature revealed intricate miRNAs-mRNAs co-regulation networks in which the anti-apoptotic XIAP, API5, BCL2, and AKT1 genes were the major targets of the set of six-miRNAs. Of these, we focused in the study of functional relationships between miR-643, upregulated at 45 min interaction, and its predicted target X-linked inhibitor of apoptosis protein (XIAP). Interestingly, interplay of amoeba with SW-480 cells resulted in downregulation of XIAP consistent with apoptosis activation. More importantly, loss of function studies using antagomiRs showed that forced inhibition of miR-643 leads to restoration of XIAP levels and suppression of both apoptosis and caspases-3 and -9 activation. Congruently, mechanistic studies using luciferase reporter assays confirmed that miR-643 exerts a postranscripcional negative regulation of XIAP by targeting its 3'-UTR indicating that it's a downstream effector. In summary, we provide novel lines of evidence suggesting that early-branched eukaryote E. histolytica may promote apoptosis of human colon cells by modulating, in part, the host microRNome which highlight an unexpected role for miRNA-643/XIAP axis in the host cellular response to parasites infection.
Keywords: Entamoeba histolytica; SW480; XIAP; apoptosis; microRNAs.
Figures
Similar articles
-
miR-142-5p enhances cisplatin-induced apoptosis in ovarian cancer cells by targeting multiple anti-apoptotic genes.Biochem Pharmacol. 2019 Mar;161:98-112. doi: 10.1016/j.bcp.2019.01.009. Epub 2019 Jan 11. Biochem Pharmacol. 2019. PMID: 30639456
-
MicroRNA profiling reveals dysregulated microRNAs and their target gene regulatory networks in cemento-ossifying fibroma.J Oral Pathol Med. 2018 Jan;47(1):78-85. doi: 10.1111/jop.12650. Epub 2017 Nov 1. J Oral Pathol Med. 2018. PMID: 29032608
-
microRNA-137 promotes apoptosis in ovarian cancer cells via the regulation of XIAP.Br J Cancer. 2017 Jan 3;116(1):66-76. doi: 10.1038/bjc.2016.379. Epub 2016 Nov 22. Br J Cancer. 2017. PMID: 27875524 Free PMC article.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Recent insights into Entamoeba development: identification of transcriptional networks associated with stage conversion.Int J Parasitol. 2009 Jan;39(1):41-7. doi: 10.1016/j.ijpara.2008.09.004. Epub 2008 Oct 4. Int J Parasitol. 2009. PMID: 18938171 Free PMC article. Review.
Cited by
-
The effect of Entamoeba histolytica lectin antigen and microRNA-643 on the development of microsatellite instability (MSI) in colorectal adenocarcinoma.BMC Cancer. 2025 Apr 10;25(1):663. doi: 10.1186/s12885-025-13472-x. BMC Cancer. 2025. PMID: 40211226 Free PMC article.
-
Acute Infectious Gastroenteritis: The Causative Agents, Omics-Based Detection of Antigens and Novel Biomarkers.Children (Basel). 2021 Dec 2;8(12):1112. doi: 10.3390/children8121112. Children (Basel). 2021. PMID: 34943308 Free PMC article. Review.
-
miRNA, New Perspective to World of Intestinal Protozoa and Toxoplasma.Acta Parasitol. 2024 Sep;69(3):1690-1703. doi: 10.1007/s11686-024-00888-x. Epub 2024 Aug 19. Acta Parasitol. 2024. PMID: 39158784 Review.
-
Human microRNAs in host-parasite interaction: a review.3 Biotech. 2020 Dec;10(12):510. doi: 10.1007/s13205-020-02498-6. Epub 2020 Nov 5. 3 Biotech. 2020. PMID: 33178551 Free PMC article. Review.
-
MicorRNA-148b inhibits cell proliferation and facilitates cell apoptosis by regulating DNA Methyltransferase 1 in endometrial cancer.Transl Cancer Res. 2020 Feb;9(2):1100-1112. doi: 10.21037/tcr.2019.12.79. Transl Cancer Res. 2020. PMID: 35117454 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

