Clinically-indicated replacement versus routine replacement of peripheral venous catheters
- PMID: 30671926
- PMCID: PMC6353131
- DOI: 10.1002/14651858.CD007798.pub5
Clinically-indicated replacement versus routine replacement of peripheral venous catheters
Abstract
Background: US Centers for Disease Control guidelines recommend replacement of peripheral intravenous catheters (PIVC) no more frequently than every 72 to 96 hours. Routine replacement is thought to reduce the risk of phlebitis and bloodstream infection. Catheter insertion is an unpleasant experience for patients and replacement may be unnecessary if the catheter remains functional and there are no signs of inflammation or infection. Costs associated with routine replacement may be considerable. This is the third update of a review first published in 2010.
Objectives: To assess the effects of removing peripheral intravenous catheters when clinically indicated compared with removing and re-siting the catheter routinely.
Search methods: The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase and CINAHL and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 18 April 2018. We also undertook reference checking, and contacted researchers and manufacturers to identify additional studies.
Selection criteria: We included randomised controlled trials that compared routine removal of PIVC with removal only when clinically indicated, in hospitalised or community-dwelling patients receiving continuous or intermittent infusions.
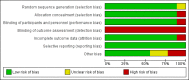
Data collection and analysis: Three review authors independently reviewed trials for inclusion, extracted data, and assessed risk of bias using Cochrane methods. We used GRADE to assess the overall evidence certainty.
Main results: This update contains two new trials, taking the total to nine included studies with 7412 participants. Eight trials were conducted in acute hospitals and one in a community setting. We rated the overall certainty of evidence as moderate for most outcomes, due to serious risk of bias for unblinded outcome assessment or imprecision, or both. Because outcome assessment was unblinded in all of the trials, none met our criteria for high methodological quality.Primary outcomesSeven trials (7323 participants), assessed catheter-related bloodstream infection (CRBSI). There is no clear difference in the incidence of CRBSI between the clinically indicated (1/3590) and routine change (2/3733) groups (risk ratio (RR) 0.61, 95% confidence interval (CI) 0.08 to 4.68), low-certainty evidence (downgraded twice for serious imprecision).All trials reported incidence of thrombophlebitis and we combined the results from seven of these in the analysis (7323 participants). We excluded two studies in the meta-analysis because they contributed to high heterogeneity. There is no clear difference in the incidence of thrombophlebitis whether catheters were changed according to clinical indication or routinely (RR 1.07, 95% CI 0.93 to 1.25; clinically indicated 317/3590; 3-day change 307/3733, moderate-certainty evidence, downgraded once for serious risk of bias). The result was unaffected by whether the infusion was continuous or intermittent. Six trials provided thrombophlebitis rates by number of device days (32,709 device days). There is no clear difference between groups (RR 0.90, 95% CI 0.76 to 1.08; clinically indicated 248/17,251; 3-day change 236/15,458; moderate-certainty evidence, downgraded once for serious risk of bias).One trial (3283 participants), assessed all-cause blood stream infection (BSI). We found no clear difference in the all-cause BSI rate between the two groups (RR 0.47, 95% CI 0.15 to 1.53; clinically indicated: 4/1593 (0.02%); routine change 9/1690 (0.05%); moderate-certainty evidence, downgraded one level for serious imprecision).Three trials (4244 participants), investigated costs; clinically indicated removal probably reduces device-related costs by approximately AUD 7.00 compared with routine removal (MD -6.96, 95% CI -9.05 to -4.86; moderate-certainty evidence, downgraded once for serious risk of bias).Secondary outcomesSix trials assessed infiltration (7123 participants). Routine replacement probably reduces infiltration of fluid into surrounding tissues compared with a clinically indicated change (RR 1.16 (95% CI 1.06 to 1.26; routine replacement 747/3638 (20.5%); clinically indicated 834/3485 (23.9%); moderate-certainty evidence, downgraded once for serious risk of bias).Meta-analysis of seven trials (7323 participants), found that rates of catheter failure due to blockage were probably lower in the routine-replacement group compared to the clinically indicated group (RR 1.14, 95% CI 1.01 to 1.29; routine-replacement 519/3733 (13.9%); clinically indicated 560/3590 (15.6%); moderate-certainty evidence, downgraded once for serious risk of bias).Four studies (4606 participants), reported local infection rates. It is uncertain if there are differences between groups (RR 4.96, 95% CI 0.24 to 102.98; clinically indicated 2/2260 (0.09%); routine replacement 0/2346 (0.0%); very low-certainty evidence, downgraded one level for serious risk of bias and two levels for very serious imprecision).One trial (3283 participants), found no clear difference in the incidence of mortality when clinically indicated removal was compared with routine removal (RR 1.06, 95% CI 0.27 to 4.23; low-certainty evidence, downgraded two levels for very serious imprecision).One small trial (198 participants) reported no clear difference in device-related pain between clinically indicated and routine removal groups (MD -0.60, 95% CI -1.44 to 0.24; low-certainty evidence, downgraded one level for serious risk of bias and one level for serious imprecision).The pre-planned outcomes 'number of catheter re-sites per patient', and 'satisfaction' were not reported by any studies included in this review.
Authors' conclusions: There is moderate-certainty evidence of no clear difference in rates of CRBSI, thrombophlebitis, all-cause BSI, mortality and pain between clinically indicated or routine replacement of PIVC. We are uncertain if local infection is reduced or increased when catheters are changed when clinically indicated. There is moderate-certainty evidence that infiltration and catheter blockage is probably lower when PIVC are changed routinely; and moderate-certainty evidence that clinically indicated removal probably reduces device-related costs. The addition of two new trials for this update found no further evidence to support changing catheters every 72 to 96 hours. Healthcare organisations may consider changing to a policy whereby catheters are changed only if there is a clinical indication to do so, for example, if there were signs of infection, blockage or infiltration. This would provide significant cost savings, spare patients the unnecessary pain of routine re-sites in the absence of clinical indications and would reduce time spent by busy clinicians on this intervention. To minimise PIVC-related complications, staff should inspect the insertion site at each shift change and remove the catheter if signs of inflammation, infiltration, occlusion, infection or blockage are present, or if the catheter is no longer needed for therapy.
Conflict of interest statement
JW: none known SO: none known CR: CR's employer has received unrestricted research grants in aid from manufacturers of peripheral intravenous catheters and products, on her behalf for academic research projects. CR's employer has also received competitively awarded research grants through professional bodies where the funds came from commercial suppliers. These sponsors (3M, Adhezion, Angiodynamics, BD‐Bard, Baxter, Medtronic, Centurion Medical, Entrotech Life Sciences, Cook, Smiths Medical) had no involvement in the study design, execution, analysis or publication of these projects, had no control over the projects, and they were unrelated to the topic of this review. CR is an academic researcher and her employer has received funding on her behalf for her to provide consultancies (expert advice, advisory panel participation, and educational lectures about her research at professional symposia and other events) from ResQDevices, Smiths Medical and BD‐Bard. NM: NM's employer has received unrestricted grants in aid from manufacturers of peripheral intravenous catheters and products, on her behalf for academic research projects. These sponsors (3M, Adhezion, BD, Centurion Medical Products, Cook Medical, Entrotech, Teleflex) had no involvement in the study design, execution, analysis or publication of these projects and they were unrelated to the topic of this review. NM is an academic researcher and her employer has received funding on her behalf for her to provide expert advice, or educational lectures on her research at professional symposia and other events from BD and Hospira.
Some of the review authors were also the investigators on several of the included trials, To eliminate any potential for a conflict of interest, assessment was allocated to a review author who was not an investigator.
Figures


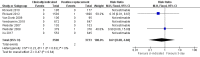












Update of
-
Clinically-indicated replacement versus routine replacement of peripheral venous catheters.Cochrane Database Syst Rev. 2015 Aug 14;(8):CD007798. doi: 10.1002/14651858.CD007798.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Jan 23;1:CD007798. doi: 10.1002/14651858.CD007798.pub5. PMID: 26272489 Updated.
Similar articles
-
Clinically-indicated replacement versus routine replacement of peripheral venous catheters.Cochrane Database Syst Rev. 2015 Aug 14;(8):CD007798. doi: 10.1002/14651858.CD007798.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Jan 23;1:CD007798. doi: 10.1002/14651858.CD007798.pub5. PMID: 26272489 Updated.
-
Clinically-indicated replacement versus routine replacement of peripheral venous catheters.Cochrane Database Syst Rev. 2013 Apr 30;(4):CD007798. doi: 10.1002/14651858.CD007798.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2015 Aug 14;(8):CD007798. doi: 10.1002/14651858.CD007798.pub4. PMID: 23633346 Updated.
-
Clinically-indicated replacement versus routine replacement of peripheral venous catheters.Cochrane Database Syst Rev. 2010 Mar 17;(3):CD007798. doi: 10.1002/14651858.CD007798.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Apr 30;(4):CD007798. doi: 10.1002/14651858.CD007798.pub3. PMID: 20238356 Updated.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Negative pressure wound therapy for surgical wounds healing by primary closure.Cochrane Database Syst Rev. 2019 Mar 26;3(3):CD009261. doi: 10.1002/14651858.CD009261.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD009261. doi: 10.1002/14651858.CD009261.pub5. PMID: 30912582 Free PMC article. Updated.
Cited by
-
Care of peripheral intravenous catheters in three hospitals in Spain: Mapping clinical outcomes and implementation of clinical practice guidelines.PLoS One. 2020 Oct 2;15(10):e0240086. doi: 10.1371/journal.pone.0240086. eCollection 2020. PLoS One. 2020. PMID: 33007001 Free PMC article.
-
Prevention and Treatment of Phlebitis Secondary to the Insertion of a Peripheral Venous Catheter: A Scoping Review from a Nursing Perspective.Healthcare (Basel). 2021 May 19;9(5):611. doi: 10.3390/healthcare9050611. Healthcare (Basel). 2021. PMID: 34069674 Free PMC article.
-
Risk Factors for Vascular Catheter Infections-Findings of a Point-Prevalence Study in 78 Hospitals.Dtsch Arztebl Int. 2021 Jul 26;118(29-30):503-504. doi: 10.3238/arztebl.m2021.0204. Dtsch Arztebl Int. 2021. PMID: 34526213 Free PMC article. No abstract available.
-
A Multicentric, Prospective, Observational, Single-Arm Registry Study to Assess the Clinical Safety and Effectiveness of Thrombophob Ointment (Heparin Sodium + Benzyl Nicotinate) in Indian Patients With Thrombophlebitis.Cureus. 2024 Feb 19;16(2):e54436. doi: 10.7759/cureus.54436. eCollection 2024 Feb. Cureus. 2024. PMID: 38510884 Free PMC article.
-
Comparison of Routine Replacement With Clinically Indicated Replacement of Peripheral Intravenous Catheters.JAMA Intern Med. 2021 Nov 1;181(11):1471-1478. doi: 10.1001/jamainternmed.2021.5345. JAMA Intern Med. 2021. PMID: 34533191 Free PMC article.
References
References to studies included in this review
Barker 2004 {published and unpublished data}
Nishanth 2009 {published data only}
-
- Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re‐siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. The International Medical Journal of India 2009;22(2):60‐2. - PubMed
Rickard 2010 {published and unpublished data}
Rickard 2012 {published and unpublished data}
-
- Rickard CM. Clinically indicated and routine replacement of peripheral IV catheters did not differ for phlebitis. Annals of Internal Medicine 2013;158:JC8. Ref ID:81. - PubMed
-
- Rickard CM, Webster J, Wallis MC, Marsh N, McGrail MR, French V, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised equivalence trial. Lancet 2012;380(9847):1066‐74. - PubMed
-
- Tuffaha HW, Rickard CM, Webster J, Marsh N, Gordon L, Wallis M, et al. Cost‐effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Applied Economics and Health Policy 2014;12:51‐8. - PubMed
Van Donk 2009 {published and unpublished data}
-
- Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infection Control and Hospital Epidemiology 2009;30(9):915‐7. - PubMed
Vendramim 2018 {unpublished data only}
-
- Vendramim P. ResPeCt‐ removal of peripheral intravenous catheters according to clinical signs or every 96 hours: randomized, controlled and non‐inferiority trial. Unpublished (final communication with trial author 1 August 2018).
Webster 2007 {published and unpublished data}
-
- Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a research base for intravenous peripheral cannula re‐sites (DRIP trial). A randomised controlled trial of hospital in‐patients. International Journal of Nursing Studies 2007;44(5):664‐71. - PubMed
Webster 2008 {published and unpublished data}
Xu 2017 {published data only}
-
- Xu L, Hu Y, Huang X, Fu J, Zhang J. Clinically indicated replacement versus routine replacement of peripheral venous catheters in adults: a nonblinded, cluster‐randomized trial in China. International Journal of Nursing Practice 2017;23:doi: 10.1111/ijn.12595. - PubMed
References to studies excluded from this review
Arnold 1977 {published data only}
-
- Arnold RE, Elliot EK, Holmes BH. The importance of frequent examination of infusion sites in preventing postinfusion phlebitis. Surgery, Gynecology and Obstetrics 1977;145(1):19‐20. - PubMed
Cobb 1992 {published data only}
-
- Cobb DK, High KP, Sawyer RG, Sable CA, Adams RB, Lindley DA, et al. A controlled trial of scheduled replacement of central venous and pulmonary‐artery catheters. New England Journal of Medicine 1992;327(15):1062‐8. - PubMed
Eyer 1990 {published data only}
-
- Eyer S, Brummitt C, Crossley K, Siegel R, Cerra F. Catheter‐related sepsis: prospective, randomized study of three methods of long‐term catheter maintenance. Critical Care Medicine 1990;18(10):1073‐9. - PubMed
Haddad 2006 {published data only}
-
- Haddad FG, Waked CH, Zein EF. Peripheral venous catheter inflammation. A randomized prospective trial. Le Journal Médical Libanais 2006;54:139‐45. - PubMed
Kerin 1991 {published data only}
-
- Kerin MJ, Pickford IR, Jaeger H, Couse NF, Mitchell CJ, Macfie J. A prospective and randomised study comparing the incidence of infusion phlebitis during continuous and cyclic peripheral parenteral nutrition. Clinical Nutrition 1991;10(6):315‐9. - PubMed
May 1996 {published data only}
-
- May J, Murchan P, MacFie J, Sedman P, Donat P, Palmer D, et al. Prospective study of the aetiology of infusion phlebitis and line failure during peripheral parenteral nutrition. British Journal of Surgery 1996;83(8):1091‐4. - PubMed
Nakae 2010 {published data only}
-
- Nakae H, Igarashi T, Tajimi K. Catheter‐related infections via temporary vascular access catheters: a randomized prospective study. Artificial Organs 2010;34(3):E72‐6. - PubMed
Panadero 2002 {published data only}
-
- Panadero A, Iohom G, Taj J, Mackay N, Shorten G. A dedicated intravenous cannula for postoperative use. Effect on incidence and severity of phlebitis. Anaesthesia 2002;57(9):921‐5. - PubMed
Rijnders 2004 {published data only}
-
- Rijnders BJ, Peetermans WE, Verwaest C, Wilmer A, Wijngaerden E. Watchful waiting versus immediate catheter removal in ICU patients with suspected catheter‐related infection: a randomized trial. Intensive Care Medicine 2004;30(6):1073‐80. - PubMed
References to studies awaiting assessment
Chin 2018 {published data only}
-
- Chin LY, Walsh TA, Haltren K, Hayden L, Davies‐Tuck M, Malhotra A. Elective replacement of intravenous cannula in newborn infants: a randomised control trial. Journal of Paediatrics and Child Health 2018;54(Suppl 1):12.
Additional references
Abolfotouh 2014
Bolton 2015
-
- Bolton D. Clinically indicated replacement of peripheral cannulas. British Journal of Nursing 2015;24:S4‐S12. - PubMed
Bregenzer 1998
-
- Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary?. Archives of Internal Medicine 1998;158:51‐6. - PubMed
Catney 2001
-
- Catney MR, Hillis S, Wakefield B, Simpson L, Domino L, Keller S, et al. Relationship between peripheral intravenous catheter dwell time and the development of phlebitis and infiltration. Journal of Infusion Nursing 2001;24(5):332‐41. - PubMed
Cornely 2002
-
- Cornely OA, Bethe U, Pauls R, Waldschmidt D. Peripheral Teflon catheters: factors determining incidence of phlebitis and duration of cannulation. Infection Control and Hospital Epidemiology 2002;23:249‐53. - PubMed
De Vries 2016
-
- Vries M, Valentine M, Mancos P. Protected clinical indication of peripheral intravenous lines: successful implementation. Journal of the Association of Vascular Access 2016;21:89‐92.
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook.
Everitt 1997
-
- Everitt NJ, Krupowicz DW, Evans JA, McMahon MJ. Ultrasonographic investigation of the pathogenesis of infusion thrombophlebitis. British Journal of Surgery 1997;84:642‐5. - PubMed
Gahlot 2014
Gorski 2016
-
- Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. Journal of Infusion Nursing 2016;39:S91.
Hadaway 2012
-
- Hadaway L. Short peripheral intravenous catheters and infections. Journal of Infusion Nursing 2012;35(4):230‐40. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June2017), Cochrane, 2017. Available from training.cochrane.org/handbook.
Ho 2011
-
- Ho KHM, Cheung DSK. Guidelines on timing in replacing peripheral intravenous catheters. Journal of Clinical Nursing 2011;21(11‐12):1499‐506. - PubMed
Homer 1998
-
- Homer LD, Holmes KR. Risks associated with 72‐ and 96‐hour peripheral intravenous catheter dwell times. Journal of Intravenous Nursing 1998;21:301‐5. - PubMed
Infusion Nurses Society 2011
-
- Infusion Nurses Society. Infusion Nursing Standards of Practice. Journal of Infusion Nursing 2011;34(1S):S57.
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
Loveday 2014
Maddox 1977
-
- Maddox RR, Rush DR, Rapp RP, Foster TS, Mazella V, McKean HE. Double‐blind study to investigate methods to prevent cephalothin‐induced phlebitis. American Journal of Hospital Pharmacy 1977;34:29‐34. - PubMed
Maki 1991
-
- Maki DG, Ringer M. Risk factors for infusion‐related phlebitis with small peripheral venous catheters. A randomized controlled trial. Annals of Internal Medicine 1991;114:845‐54. - PubMed
Maki 2006
-
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clinic Proceedings 2006;81(9):1159‐71. - PubMed
Maki 2008
Marsh 2018a
-
- Marsh N, Webster J, Ullman A, Mihala G, Cooke M, Rickard CM. The incidence of peripheral intravenous catheter failure and complications within the adult population: a systematic review. BMJ 2018; Vol. (in editorial process).
Marsh 2018b
-
- Marsh N, Webster J, Larsen E, Cooke M, Mihala G, Rickard C. Observational study of peripheral intravenous catheter outcomes in adult hospitalized patients: a multivariable analysis of peripheral intravenous catheter failure. Journal of Hospital Medicine 2018;13:83‐9. - PubMed
Mermel 2017
-
- Mermel LA. Short‐term peripheral venous catheter‐related blood stream infection. Clinical Infectious Diseases 2017;65(10):1757‐62. - PubMed
Monreal 1999
-
- Monreal M, Quilez F, Rey‐Joly C, Vega J, Torres T, Valero P, et al. Infusion phlebitis in patients with acute pneumonia: a prospective study. Chest 1999;115:1576‐80. - PubMed
Morrison 2015
-
- Morrison K, Holt KE. The effectiveness of clinically indicated replacement of peripheral intravenous catheters: an evidence review with implications for clinical practice. Worldviews Evidence Based Nursing 2015;12:187‐98. - PubMed
O'Grady 2011
-
- O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. 2011 Guidelines for the prevention of intravascular catheter‐related infections. (accessed 23 August 2018).
Patel 2017
-
- Patel SA, Alebich MM, Feldman LS. Routine replacement of peripheral intravenous catheters. Journal of Hospital Medicine 2017;12:42‐5. - PubMed
Ray‐Barruel 2014
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rickard 2018
-
- Rickard CM, Webster J, Runnegar N, Larsen E, McGrail MR, Fullerton F, et al. Dressings and securements for the prevention of peripheral intravenous catheter failure in adults (SAVE): a pragmatic,randomised controlled, superiority trial. Lancet 2018;392:419–30. [DOI: 10.1016/S0140-6736(18)31380-1] - DOI - PubMed
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook.
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook.
Tan 2016
Tuffaha 2014a
-
- Tuffaha HW, Rickard CM, Webster J, Marsh N, Gordon L, Wallis M, et al. Cost‐effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Applied Economics and Health Policy 2014;12:51‐8. - PubMed
Tuffaha 2014b
-
- Tuffaha HW, Rickard CM, Inwood S, Gordon L, Scuffham P. The epic3 recommendation that clinically indicated replacement of peripheral venous catheters is safe and cost‐saving: how much would the NHS save?. Journal of Hospital Infection 2014;87(3):183‐4. - PubMed
Uslusoy 2008
-
- Uslusoy E, Mete S. Predisposing factors to phlebitis in patients with peripheral intravenous catheters: a descriptive study. Journal of the American Academy of Nurse Practitioners 2008;20:172‐80. - PubMed
White 2001
-
- White SA. Peripheral intravenous therapy‐related phlebitis rates in an adult population. Journal of Intravenous Nursing 2001;24:19‐24. - PubMed
References to other published versions of this review
Webster 2009
Webster 2010
Webster 2013
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

