Restabilization treatment after intravenous immunoglobulin withdrawal in chronic inflammatory demyelinating polyneuropathy: Results from the pre-randomization phase of the Polyneuropathy And Treatment with Hizentra study
- PMID: 30672067
- PMCID: PMC6593755
- DOI: 10.1111/jns.12303
Restabilization treatment after intravenous immunoglobulin withdrawal in chronic inflammatory demyelinating polyneuropathy: Results from the pre-randomization phase of the Polyneuropathy And Treatment with Hizentra study
Abstract
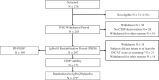
In patients with chronic inflammatory demyelinating polyneuropathy (CIDP), intravenous immunoglobulin (IVIG) is recommended to be periodically reduced to assess the need for ongoing therapy. However, little is known about the effectiveness of restabilization with IVIG in patients who worsen after IVIG withdrawal. In the Polyneuropathy And Treatment with Hizentra (PATH) study, the pre-randomization period included sudden stopping of IVIG followed by 12 weeks of observation. Those deteriorating were then restabilized with IVIG. Of 245 subjects who stopped IVIG, 28 did not show signs of clinical deterioration within 12 weeks. Two hundred and seven received IVIG restabilization with an induction dose of 2 g/kg bodyweight (bw) IgPro10 (Privigen, CSL Behring, King of Prussia, Pennsylvania) and maintenance doses of 1 g/kg bw every 3 weeks for up to 13 weeks. Signs of clinical improvement were seen in almost all (n = 188; 91%) subjects. During IVIG restabilization, 35 subjects either did not show CIDP stability (n = 21, analyzed as n = 22 as an additional subject was randomized in error) or withdrew for other reasons (n = 14). Of the 22 subjects who did not achieve clinical stability, follow-up information in 16 subjects after an additional 4 weeks was obtained. Nine subjects were reported to have improved, leaving a maximum of 27 subjects (13%) who either showed no signs of clinical improvement during the restabilization phase and 4 weeks post-study or withdrew for other reasons. In conclusion, sudden IVIG withdrawal was effective in detecting ongoing immunoglobulin G dependency with a small risk for subjects not returning to their baseline 17 weeks after withdrawal.
Keywords: Privigen; chronic inflammatory demyelinating polyneuropathy (CIDP); inflammatory neuropathy cause and treatment (INCAT); intravenous immunoglobulin (IVIG); polyneuropathy and treatment with Hizentra (PATH).
© 2019 CSL Behring. Journal of the Peripheral Nervous System published by Wiley Periodicals, Inc. on behalf of Peripheral Nerve Society.
Figures


References
-
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of paraproteinemic demyelinating neuropathies . Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society–first revision. J Peripher Nerv Syst. 2010;15:185‐195. - PubMed
-
- Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2009;21:CD001797. - PubMed
-
- van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurol. 2018;17:35‐46. - PubMed
-
- RMC Trial Group . Randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC trial): a pilot, multicentre study. Lancet Neurol. 2009;8:158‐164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
- Andalusian Health Repository - access to free full text
- Archivio Istituzionale della Ricerca Unimi - Access Free Full Text
- Digital CSIC Spanish National Research Council - Access Free Full Text
- Europe PubMed Central
- Ovid Technologies, Inc.
- PubMed Central
- University of Turin Instituional Repository AperTO - Free full text
- Wiley

