Mutual regulation of MDM4 and TOP2A in cancer cell proliferation
- PMID: 30672125
- PMCID: PMC6487731
- DOI: 10.1002/1878-0261.12457
Mutual regulation of MDM4 and TOP2A in cancer cell proliferation
Retraction in
-
RETRACTION: Mutual Regulation of MDM4 and TOP2A in Cancer Cell Proliferation.Mol Oncol. 2025 Jan;19(1):262. doi: 10.1002/1878-0261.13776. Epub 2024 Dec 3. Mol Oncol. 2025. PMID: 39627951 Free PMC article.
Abstract
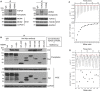
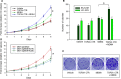
MDM4 and topoisomerase IIα (TOP2A) are overexpressed in various human cancers. MDM4 acts as an oncoprotein which promotes cancer progression by inhibiting tumor suppressor p53. As a DNA replication- and cell division-regulating enzyme, TOP2A is the main target of many anticancer therapy regimens; however, the exact role of TOP2A in cancer remains elusive. Herein, we report that MDM4 and TOP2A bind to each other and are mutually upregulated at the post-translational level, leading to TOP2A protein stabilization, inhibition of p53, and increased tumor-cell proliferation. We demonstrate that the C-terminal region (CTR) of TOP2A binds to a unique sequence (residues: 188-238) of MDM4, which contains an auto-inhibitory segment regulating the MDM4-p53 interaction. TOP2A binding in turn activates MDM4 for p53 binding, resulting in enhanced inhibition of p53 and cancer cell proliferation. Conversely, binding of the MDM4 sequence to the CTR of TOP2A stabilizes TOP2A protein, leading to increased TOP2A protein expression. These results reveal novel functions of MDM4 and TOP2A as well as their interactions in oncogenesis, suggesting that inhibition of the MDM4-TOP2A interaction may represent a novel strategy in specifically and simultaneously targeting TOP2A and MDM4 for cancer treatment.
Keywords: MDM4; TOP2A; cancer cell proliferation; p53.
© 2019 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

