Consistency in microbiomes in cultures of Alexandrium species isolated from brackish and marine waters
- PMID: 30672139
- PMCID: PMC6563467
- DOI: 10.1111/1758-2229.12736
Consistency in microbiomes in cultures of Alexandrium species isolated from brackish and marine waters
Abstract
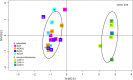
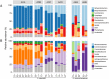
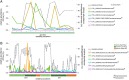
Phytoplankton and bacteria interactions have a significant role in aquatic ecosystem functioning. Associations can range from mutualistic to parasitic, shaping biogeochemical cycles and having a direct influence on phytoplankton growth. How variations in phenotype and sampling location, affect the phytoplankton microbiome is largely unknown. A high-resolution characterization of the bacterial community in cultures of the dinoflagellate Alexandrium was performed on strains isolated from different geographical locations and at varying anthropogenic impact levels. Microbiomes of Baltic Sea Alexandrium ostenfeldii isolates were dominated by Betaproteobacteria and were consistent over phenotypic and genotypic Alexandrium strain variation, resulting in identification of an A. ostenfeldii core microbiome. Comparisons with in situ bacterial communities showed that taxa found in this A. ostenfeldii core were specifically associated to dinoflagellate dynamics in the Baltic Sea. Microbiomes of Alexandrium tamarense and minutum, isolated from the Mediterranean Sea, differed from those of A. ostenfeldii in bacterial diversity and composition but displayed high consistency, and a core set of bacterial taxa was identified. This indicates that Alexandrium isolates with diverse phenotypes host predictable, species-specific, core microbiomes reflecting the abiotic conditions from which they were isolated. These findings enable in-depth studies of potential interactions occurring between Alexandrium and specific bacterial taxa.
© 2019 The Authors. Environmental Microbiology Reports published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Phylogenetic diversity and specificity of bacteria closely associated with Alexandrium spp. and other phytoplankton.Appl Environ Microbiol. 2005 Jul;71(7):3483-94. doi: 10.1128/AEM.71.7.3483-3494.2005. Appl Environ Microbiol. 2005. PMID: 16000752 Free PMC article.
-
Identification of unique microbiomes associated with harmful algal blooms caused by Alexandrium fundyense and Dinophysis acuminata.Harmful Algae. 2017 Sep;68:17-30. doi: 10.1016/j.hal.2017.07.003. Epub 2017 Jul 24. Harmful Algae. 2017. PMID: 28962978
-
Morphological, molecular, and toxin analysis of field populations of Alexandrium genus from the Argentine Sea.J Phycol. 2017 Dec;53(6):1206-1222. doi: 10.1111/jpy.12574. Epub 2017 Sep 14. J Phycol. 2017. PMID: 28793388
-
Parasites and phytoplankton, with special emphasis on dinoflagellate infections.J Eukaryot Microbiol. 2004 Mar-Apr;51(2):145-55. doi: 10.1111/j.1550-7408.2004.tb00539.x. J Eukaryot Microbiol. 2004. PMID: 15134249 Review.
-
Growth Rates of Microbes in the Oceans.Ann Rev Mar Sci. 2016;8:285-309. doi: 10.1146/annurev-marine-122414-033938. Epub 2015 Jul 17. Ann Rev Mar Sci. 2016. PMID: 26195108 Review.
Cited by
-
Identification and implications of a core bacterial microbiome in 19 clonal cultures laboratory-reared for months to years of the cosmopolitan dinoflagellate Karlodinium veneficum.Front Microbiol. 2022 Aug 4;13:967610. doi: 10.3389/fmicb.2022.967610. eCollection 2022. Front Microbiol. 2022. PMID: 36033882 Free PMC article.
-
Microbiome and environment explain the absence of correlations between consumers and their diet in Bornean microsnails.Ecology. 2021 Feb;102(2):e03237. doi: 10.1002/ecy.3237. Epub 2021 Jan 18. Ecology. 2021. PMID: 33098661 Free PMC article.
-
Algal blooms in the ocean: hot spots for chemically mediated microbial interactions.Nat Rev Microbiol. 2024 Mar;22(3):138-154. doi: 10.1038/s41579-023-00975-2. Epub 2023 Oct 13. Nat Rev Microbiol. 2024. PMID: 37833328 Review.
-
Diverse interactions between bacteria and microalgae: A review for enhancing harmful algal bloom mitigation and biomass processing efficiency.Heliyon. 2024 Aug 24;10(17):e36503. doi: 10.1016/j.heliyon.2024.e36503. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39286093 Free PMC article. Review.
-
Functional diversity of bacterial microbiota associated with the toxigenic benthic dinoflagellate Prorocentrum.PLoS One. 2024 Jul 16;19(7):e0306108. doi: 10.1371/journal.pone.0306108. eCollection 2024. PLoS One. 2024. PMID: 39012861 Free PMC article.
References
-
- Amin, S.A. , Hmelo, L.R. , van Tol, H.M. , Durham, B.P. , Carlson, L.T. , Heal, K.R. , et al (2015) Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 522: 98–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

