Inhibition of multiple voltage-gated calcium channels may contribute to spinally mediated analgesia by epipregnanolone in a rat model of surgical paw incision
- PMID: 30672394
- PMCID: PMC6380214
- DOI: 10.1080/19336950.2018.1564420
Inhibition of multiple voltage-gated calcium channels may contribute to spinally mediated analgesia by epipregnanolone in a rat model of surgical paw incision
Abstract
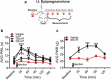
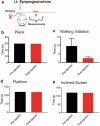
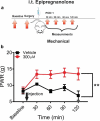
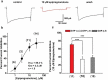
Voltage-activated calcium channels play an important role in excitability of sensory nociceptive neurons in acute and chronic pain models. We have previously shown that low-voltage-activated calcium channels, or T-type channels (T-channels), increase excitability of sensory neurons after surgical incision in rats. We have also found that endogenous 5β-reduced neuroactive steroid epipregnanolone [(3β,5β)-3-hydroxypregnan-20-one] blocked isolated T-currents in dorsal root ganglion (DRG) cells in vitro, and reduced nociceptive behavior in vivo, after local intraplantar application into the foot pads of heathy rats and mice. Here, we investigated if epipregnanolone exerts an antinociceptive effect after intrathecal (i.t.) application in healthy rats, as well as an antihyperalgesic effect in a postsurgical pain model. We also studied if this endogenous neurosteroid blocks currents originating from high voltage-activated (HVA) calcium channels in rat sensory neurons. In in vivo studies, we found that epipregnanolone alleviated thermal and mechanical nociception in healthy rats after i.t. administration without affecting their sensory-motor abilities. Furthermore, epipregnanolone effectively reduced mechanical hyperalgesia after i.t application in rats after surgery. In subsequent in vitro studies, we found that epipregnanolone blocked isolated HVA currents in nociceptive sensory neurons with an IC50 of 3.3 μM in a G-protein-dependent fashion. We conclude that neurosteroids that have combined inhibitory effects on T-type and HVA calcium currents may be suitable for development of novel pain therapies during the perioperative period.
Keywords: Neurosteroids; calcium; hyperalgesia; low voltage-activated calcium channels.
Figures
Similar articles
-
Inhibition of CaV3.2 T-type calcium channels in peripheral sensory neurons contributes to analgesic properties of epipregnanolone.Psychopharmacology (Berl). 2014 Sep;231(17):3503-3515. doi: 10.1007/s00213-014-3588-0. Epub 2014 May 7. Psychopharmacology (Berl). 2014. PMID: 24800894 Free PMC article.
-
New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5alpha-reduced neuroactive steroids.Pain. 2005 Apr;114(3):429-443. doi: 10.1016/j.pain.2005.01.009. Pain. 2005. PMID: 15777868
-
5beta-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo.Mol Pharmacol. 2004 Nov;66(5):1223-35. doi: 10.1124/mol.104.002402. Epub 2004 Jul 27. Mol Pharmacol. 2004. PMID: 15280444
-
Targeting of CaV3.2 T-type calcium channels in peripheral sensory neurons for the treatment of painful diabetic neuropathy.Pflugers Arch. 2014 Apr;466(4):701-6. doi: 10.1007/s00424-014-1452-z. Epub 2014 Jan 31. Pflugers Arch. 2014. PMID: 24482063 Review.
-
Recent advances in the development of T-type calcium channel blockers for pain intervention.Br J Pharmacol. 2018 Jun;175(12):2375-2383. doi: 10.1111/bph.13906. Epub 2017 Jul 12. Br J Pharmacol. 2018. PMID: 28608534 Free PMC article. Review.
Cited by
-
Effects of benidipine, paracetamol, and their combination on postoperative and normal tissue pain thresholds.Front Pharmacol. 2024 Jan 5;14:1326128. doi: 10.3389/fphar.2023.1326128. eCollection 2023. Front Pharmacol. 2024. PMID: 38249347 Free PMC article.
-
Different roles of T-type calcium channel isoforms in hypnosis induced by an endogenous neurosteroid epipregnanolone.Neuropharmacology. 2021 Oct 1;197:108739. doi: 10.1016/j.neuropharm.2021.108739. Epub 2021 Jul 31. Neuropharmacology. 2021. PMID: 34339750 Free PMC article.
-
Thalamic T-Type Calcium Channels as Targets for Hypnotics and General Anesthetics.Int J Mol Sci. 2022 Feb 21;23(4):2349. doi: 10.3390/ijms23042349. Int J Mol Sci. 2022. PMID: 35216466 Free PMC article. Review.
-
β-Caryophyllene Inhibits Monoacylglycerol Lipase Activity and Increases 2-Arachidonoyl Glycerol Levels In Vivo: A New Mechanism of Endocannabinoid-Mediated Analgesia?Mol Pharmacol. 2024 Jan 10;105(2):75-83. doi: 10.1124/molpharm.123.000668. Mol Pharmacol. 2024. PMID: 38195158 Free PMC article.
-
Epipregnanolone as a Positive Modulator of GABAA Receptor in Rat Cerebellar and Hippocampus Neurons.Biomolecules. 2021 May 24;11(6):791. doi: 10.3390/biom11060791. Biomolecules. 2021. PMID: 34074021 Free PMC article.
References
-
- Chapman CR, Stevens DA, Lipman AG.. Quality of postoperative pain management in American versus European institutions. J Pain Palliat Care Pharmacother. 2013. December;27(4):350–358. . - PubMed
-
- Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, et al. Procedure-specific risk factor analysis for thedevelopment of severe postoperative pain. Anesthesiology. 2014. May;120(5):1237–1245. . - PubMed
-
- Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: resultsfrom a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003. August;97(2):534–40, table of contents. PubMed PMID: 12873949 - PubMed
-
- Pavlin DJ, Chen C, Penaloza DA, et al. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg. 2002. September;95(3):627–34, table of contents. PubMed PMID: 12198050 - PubMed
-
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000. April;26(1):13–25. Review PubMed PMID: 10798388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
