Caspofungin for primary antifungal prophylaxis after T-cell-replete haploidentical stem cell transplantation with post-transplant cyclophosphamide
- PMID: 30672611
- PMCID: PMC7163667
- DOI: 10.1111/ejh.13214
Caspofungin for primary antifungal prophylaxis after T-cell-replete haploidentical stem cell transplantation with post-transplant cyclophosphamide
Abstract
Objectives: T-cell-replete haploidentical stem cell transplantation (Haplo-SCT) with post-transplant cyclophosphamide (PT-Cy) is at high risk of invasive fungal infections (IFI), and anti-mold-active drug is required for primary antifungal prophylaxis (PAP) according to international guidelines. No data are available on the efficacy of caspofungin as PAP in this setting.
Methods: Here, we report our retrospective experience with 103 consecutive patients treated with caspofungin as PAP after Haplo-SCT. Caspofungin was administered only during the pre-engraftment phase.
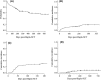
Results: Hundred-day cumulative incidence of proven-probable IFI (PP-IFI) was 8.7% and median day of onset was 19 post-SCT. No patient died of PP-IFI, and overall survival (OS) and non-relapse mortality (NRM) hazard ratio (HR) for patients experiencing IFI were 1.02 (P = 0.9) and 0.7 (P = 0.7), respectively. Three-year overall survival (OS) and 1-year non-relapse mortality (NRM) were 55% and 19%, respectively. By univariate analysis, duration of neutropenic phase and partial remission pre-transplant disease status were associated with increased incidence of IFI, but were not confirmed by multivariate analysis.
Conclusion: In summary, PAP with caspofungin is an effective strategy for preventing IFI in the context of Haplo-SCT with PT-Cy. Further efforts are required in order to identify more potent strategies able to avoid the occurrence of breakthrough infections.
Keywords: caspofungin; haploidentical stem cell transplant; primary antifungal prophylaxis.
© 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures

Similar articles
-
A reduced dose of fluconazole as primary antifungal prophylaxis is not associated with increased risk of invasive fungal infections after allogeneic stem cell transplantation from a HLA identical sibling.Transpl Infect Dis. 2018 Aug;20(4):e12906. doi: 10.1111/tid.12906. Epub 2018 May 16. Transpl Infect Dis. 2018. PMID: 29668124
-
Management of Invasive Fungal Infections in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation: The Turin Experience.Front Cell Infect Microbiol. 2022 Jan 7;11:805514. doi: 10.3389/fcimb.2021.805514. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35071052 Free PMC article.
-
Caspofungin as primary antifungal prophylaxis in stem cell transplant recipients.Pharmacotherapy. 2007 Dec;27(12):1644-50. doi: 10.1592/phco.27.12.1644. Pharmacotherapy. 2007. PMID: 18041885
-
Invasive fungal infection in haematopoietic stem cell transplant recipients: epidemiology from the transplant physician's viewpoint.Mycopathologia. 2009 Dec;168(6):283-97. doi: 10.1007/s11046-009-9196-6. Epub 2009 Apr 3. Mycopathologia. 2009. PMID: 19343534 Review.
-
Antifungal prophylaxis among allogeneic hematopoietic stem cell transplant recipients: current issues and new agents.Expert Rev Anti Infect Ther. 2006 Jun;4(3):457-68. doi: 10.1586/14787210.4.3.457. Expert Rev Anti Infect Ther. 2006. PMID: 16771622 Review.
Cited by
-
Prophylaxis for invasive fungal infection in pediatric patients with allogeneic hematopoietic stem cell transplantation.Blood Res. 2022 Mar 31;57(1):34-40. doi: 10.5045/br.2021.2021127. Blood Res. 2022. PMID: 35256547 Free PMC article.
-
Cell Wall Composition Heterogeneity between Single Cells in Aspergillus fumigatus Leads to Heterogeneous Behavior during Antifungal Treatment and Phagocytosis.mBio. 2020 May 12;11(3):e03015-19. doi: 10.1128/mBio.03015-19. mBio. 2020. PMID: 32398317 Free PMC article.
-
Population Pharmacokinetics of Caspofungin and Dosing Optimization in Children With Allogeneic Hematopoietic Stem Cell Transplantation.Front Pharmacol. 2020 Mar 2;11:184. doi: 10.3389/fphar.2020.00184. eCollection 2020. Front Pharmacol. 2020. PMID: 32194415 Free PMC article.
References
-
- Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B‐2004 study–Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45:1161‐1170. - PubMed
-
- Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant‐Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091‐1100. - PubMed
-
- Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265‐273. - PubMed
-
- Kurosawa M, Yonezumi M, Hashino S, et al. Epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int J Hematol. 2012;96:748‐757. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

