Long-term efficacy and safety of secukinumab in Japanese patients with moderate to severe plaque psoriasis: 3-year results of a double-blind extension study
- PMID: 30672623
- PMCID: PMC6590222
- DOI: 10.1111/1346-8138.14761
Long-term efficacy and safety of secukinumab in Japanese patients with moderate to severe plaque psoriasis: 3-year results of a double-blind extension study
Abstract
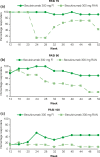
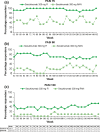
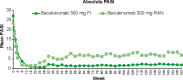
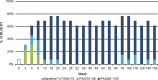
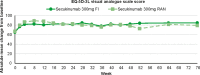
Secukinumab, a fully human monoclonal antibody neutralizing interleukin-17A, has been shown to have significant efficacy in the treatment of moderate to severe psoriasis. Long-term (3-year) efficacy and safety of secukinumab in Japanese patients with moderate to severe psoriasis were evaluated in an extension study of a large phase 3 global study (SCULPTURE). In the core study, 52 Japanese patients with 75% improvement of Psoriasis Area and Severity Index (PASI-75) response at week 12 were re-randomized to a fixed interval (FI; every 4 weeks) schedule and retreatment as needed (RAN), in which patients received placebo until start of relapse, at which time secukinumab was reinitiated. Fifty Japanese patients completed the 52-week core study, and 47 patients entered the extension study with the same double-blind regimens up to week 152. All patients in the secukinumab 300 mg FI and seven patients in 150 mg FI groups completed 3 years of treatment. PASI-90 and -100 at the end of year 3 were achieved in 69.2% and 53.8%, respectively, in 300 mg FI and 42.9% and 42.9%, respectively, in 150 mg FI, indicating high sustained response in 300 mg FI. Mean absolute PASI was continually low in 300 mg FI and numerically higher in 150 mg FI. Dermatology Life Quality Index of 0/1 was maintained by approximately two-thirds of 300 mg FI patients, and all EuroQoL 5-Dimension Health Questionnaire domain measures were also improved. FI dosing was consistently more efficacious than RAN. The safety profile of secukinumab remained favorable, with no new safety concerns identified.
Keywords: Dermatology Life Quality Index; EuroQol 5-Dimension; psoriasis; randomized controlled trial; secukinumab.
© 2019 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Figures
References
-
- Blauvelt A, Prinz JC, Gottlieb AB et al Secukinumab administration by pre‐filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol 2015; 172(2): 484–93. - PubMed
-
- Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis‐results of two phase 3 trials. N Engl J Med 2014; 371(4): 326–38. - PubMed
-
- Mrowietz U, Leonardi CL, Girolomoni G et al Secukinumab retreatment‐as‐needed versus fixed‐interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double‐blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol 2015; 73(1): 27–36. e1 - PubMed
-
- Paul C, Lacour JP, Tedremets L et al Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol 2015; 29(6): 1082–90. - PubMed
-
- van de Kerkhof PC, Griffiths CE, Reich K et al Secukinumab long‐term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol 2016; 75(1): 83–98. e4 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

