A unified model for microtubule rescue
- PMID: 30672721
- PMCID: PMC6589779
- DOI: 10.1091/mbc.E18-08-0541
A unified model for microtubule rescue
Abstract
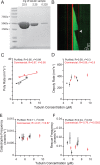
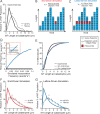
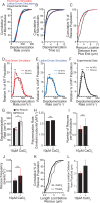
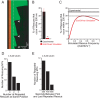
How microtubules transition from depolymerization to polymerization, known as rescue, is poorly understood. Here we examine two models for rescue: 1) an "end-driven" model in which the depolymerizing end stochastically switches to a stable state; and 2) a "lattice-driven" model in which rescue sites are integrated into the microtubule before depolymerization. We test these models using a combination of computational simulations and in vitro experiments with purified tubulin. Our findings support the "lattice-driven" model by identifying repeated rescue sites in microtubules. In addition, we discover an important role for divalent cations in determining the frequency and location of rescue sites. We use "wash-in" experiments to show that divalent cations inhibit rescue during depolymerization, but not during polymerization. We propose a unified model in which rescues are driven by embedded rescue sites in microtubules, but the activity of these sites is influenced by changes in the depolymerizing ends.
Figures

References
-
- Aher A, Akhmanova A. (2018). Tipping microtubule dynamics, one protofilament at a time. Curr Opin Cell Biol , 86–93. - PubMed
-
- Arnal I, Heichette C, Diamantopoulos GS, Chrétien D. (2004). CLIP-170/tubulin-curved oligomers coassemble at microtubule ends and promote rescues. Curr Biol , 2086–2095. - PubMed
-
- Bers DM, Patton CW, Nuccitelli R. (2010). A practical guide to the preparation of Ca2+buffers. Methods Cell Biol , 1–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

