Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D) - What We Have Learned after 40 Years of the Diagnosis of This Clinical Entity
- PMID: 30673021
- PMCID: PMC6317628
- DOI: 10.5935/abc.20180266
Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D) - What We Have Learned after 40 Years of the Diagnosis of This Clinical Entity
Erratum in
-
Erratum.Arq Bras Cardiol. 2019 Feb;112(2):214. doi: 10.5935/abc.20190019. Arq Bras Cardiol. 2019. PMID: 30785589 Free PMC article.
Abstract
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) was initially recognized as a clinical entity by Fontaine and Marcus, who evaluated a group of patients with ventricular tachyarrhythmia from a structurally impaired right ventricle (RV). Since then, there have been significant advances in the understanding of the pathophysiology, manifestation and clinical progression, and prognosis of the pathology. The identification of genetic mutations impairing cardiac desmosomes led to the inclusion of this entity in the classification of cardiomyopathies. Classically, ARVC/D is an inherited disease characterized by ventricular arrhythmias, right and / or left ventricular dysfunction; and fibro-fatty substitution of cardiomyocytes; its identification can often be challenging, due to heterogeneous clinical presentation, highly variable intra- and inter-family expressiveness, and incomplete penetrance. In the absence of a gold standard that allows the diagnosis of ARVC/D, several diagnostic categories were combined and recently reviewed for a higher diagnostic sensitivity, without compromising the specificity. The finding that electrical abnormalities, particularly ventricular arrhythmias, usually precede structural abnormalities is extremely important for risk stratification in positive genetic members. Among the complementary exams, cardiac magnetic resonance imaging (CMR) allows the early diagnosis of left ventricular impairment, even before morpho-functional abnormalities. Risk stratification remains a major clinical challenge, and antiarrhythmic drugs, catheter ablation and implantable cardioverter defibrillator are the currently available therapeutic tools. The disqualification of the sport prevents cases of sudden death because the effort can trigger not only the electrical instability, but also the onset and progression of the disease.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

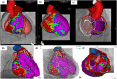
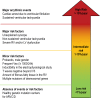
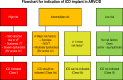
References
-
- Elias J, Tonet J, Frank R, Fontaine G. Arrhythmogenic right ventricular dysplasia. Arq Bras Cardiol. 1998;70(6):449–456. - PubMed
-
- Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65(2):384–398. - PubMed
-
- Wang W, James CA, Calkins H. Diagnostic and therapeutic strategies for arrhythmogenic right ventricular dysplasia/cardiomyopathy patient. Europace. Apr 23, 2018. [Epub ahead of print] Disponível em: https://academic.oup.com/europace/advance-article. - PMC - PubMed
-
- Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376(1):61–72. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

