Prevalence of G6PD deficiency and molecular characterization of G202A, A376G and C563T polymorphisms in newborns in Southeastern Brazil
- PMID: 30673054
- PMCID: PMC6438675
- DOI: 10.31744/einstein_journal/2019AO4436
Prevalence of G6PD deficiency and molecular characterization of G202A, A376G and C563T polymorphisms in newborns in Southeastern Brazil
Abstract
Objective: To evaluate the prevalence of G6PD deficiency and characterize G202A, A376G and C563T polymorphisms in neonates using molecular assays.
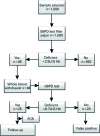
Methods: A total of one thousand samples were tested through quantitative analysis of enzyme activity, detecting 25 G6PD-deficient individuals. Patients identified as deficient were submitted to molecular analysis quantitative real-time polymerase chain reaction - (qPCR) to investigate the presence of variants associated with the deficiency.
Results: The total prevalence of G6PD deficient was 2.5%. Of the 25 samples identified as deficient, 21 were submitted to qPCR assay to analyze the presence of G202A, A376G and C563T variants. All samples showed the G202A/A376G genotype, characterizing G6PD A- phenotype.
Conclusion: The prevalence of G6PD deficiency in the present study was similar to that observed in other study populations in Brazil. Molecular analysis identified in all patients the presence of the genetic polymorphism G202A/A376G, more common in the Brazilian population with G6PD deficiency, which is directly estimated by enzyme activity level.
Objetivo: Avaliar a prevalência da deficiência de G6PD e caracterizar, por ensaios moleculares, os polimorfismos G202A, A376G e C563T em recém-nascidos.
Métodos: Foram testadas mil amostras por meio da análise quantitativa da atividade enzimática, detectando 25 portadores de deficiência de G6PD. Os pacientes identificados como deficientes foram submetidos à análise molecular reação em cadeia da polimerase em tempo real (qPCR) para pesquisa da presença das variantes associadas à deficiência.
Resultados: A prevalência total de deficientes de G6PD foi de 2,5%. Das 25 amostras identificadas como deficientes, 21 foram submetidas ao qPCR, para análise da presença das variantes G202A, A376G e C563T. Todas as amostras apresentaram o genótipo G202A/A376G, caracterizando fenótipo G6PD A-.
Conclusão: A prevalência da deficiência da G6PD no presente estudo foi semelhante à verificada em outras populações de estudo no Brasil. A análise molecular identificou em todos os pacientes a presença do polimorfismo genético G202A/A376G, mais comum na população brasileira portadora da deficiência de G6PD, que é diretamente estimada pelo nível de atividade enzimática.
Conflict of interest statement
Figures
Similar articles
-
Prevalence of glucose-6-phosphate dehydrogenase deficiency and the role of the A- variant in a Saudi population.J Int Med Res. 2014 Oct;42(5):1161-7. doi: 10.1177/0300060514531923. Epub 2014 Aug 28. J Int Med Res. 2014. PMID: 25169987
-
Glucose-6-phosphate dehydrogenase deficiency allelic variants and their prevalence in malaria patients in Eritrea.Pan Afr Med J. 2018 Sep 20;31:46. doi: 10.11604/pamj.2018.31.46.16527. eCollection 2018. Pan Afr Med J. 2018. PMID: 30918572 Free PMC article.
-
Glucose-6-phosphate dehydrogenase (G6PD) deficiency in Ethiopia: absence of common African and Mediterranean allelic variants in a nationwide study.Malar J. 2018 Oct 26;17(1):388. doi: 10.1186/s12936-018-2538-4. Malar J. 2018. PMID: 30367627 Free PMC article.
-
[Molecular identification of glucose-6-phosphate dehydrogenase (G6PD) detected in neonatal screening].Gac Med Mex. 2015 Jan-Feb;151(1):34-41. Gac Med Mex. 2015. PMID: 25739482 Spanish.
-
Molecular Characterization of Erythrocyte Glucose-6-Phosphate Dehydrogenase Deficiency in Different Ethnic Groups of Blood Donors in Mauritania.Front Biosci (Schol Ed). 2023 Sep 25;15(3):11. doi: 10.31083/j.fbs1503011. Front Biosci (Schol Ed). 2023. PMID: 37806950
References
-
- Luzzatto L. Glucose 6-phosphate dehydrogenase deficiency: from genotype to phenotype. Haematologica. 2006;91(10):1303–1306. - PubMed
-
- Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606):64–74. Review. - PubMed
-
- Luzzatto L, Mehta A. Glucose-6-phosphate dehydrogenase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editor, editors. The Metabolic and Molecular Basis of Inherited Disease. 7th. Vol. 3. New York: Mcgraw-Hill; 1995. pp. 3367–3398.
-
- Gaetani GF, Galiano S, Canepa L, Ferraris AM, Kirkman HN. Catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes. Blood. 1989;73(1):334–339. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous