Colloidal Model for the Prediction of the Extraction of Rare Earths Assisted by the Acidic Extractant
- PMID: 30673246
- PMCID: PMC6488188
- DOI: 10.1021/acs.langmuir.8b03846
Colloidal Model for the Prediction of the Extraction of Rare Earths Assisted by the Acidic Extractant
Abstract
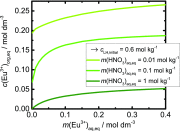
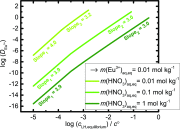
We propose the statistical thermodynamic model for the prediction of the liquid-liquid extraction efficiency in the case of rare-earth metal cations using the common bis(2-ethyl-hexyl)phosphoric acid (HDEHP) extractant. In this soft matter-based approach, the solutes are modeled as colloids. The leading terms in free-energy representation account for: the complexation, the formation of a highly curved extractant film, lateral interactions between the different extractant head groups in the film, configurational entropy of ions and water molecules, the dimerization, and the acidity of the HDEHP extractant. We provided a full framework for the multicomponent study of extraction systems. By taking into account these different contributions, we are able to establish the relation between the extraction and general complexation at any pH in the system. This further allowed us to rationalize the well-defined optimum in the extraction engineering design. Calculations show that there are multiple extraction regimes even in the case of lanthanide/acid system only. Each of these regimes is controlled by the formation of different species in the solvent phase, ranging from multiple metal cation-filled aggregates (at the low acid concentrations in the aqueous phase), to the pure acid-filled aggregates (at the high acid concentrations in the aqueous phase). These results are contrary to a long-standing opinion that liquid-liquid extraction can be modeled with only a few species. Therefore, a traditional multiple equilibria approach is abandoned in favor of polydisperse spherical aggregate formations, which are in dynamic equilibrium.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
Multicomponent Model for the Prediction of Nuclear Waste/Rare-Earth Extraction Processes.Langmuir. 2018 Sep 4;34(35):10434-10447. doi: 10.1021/acs.langmuir.8b01759. Epub 2018 Aug 24. Langmuir. 2018. PMID: 30081639 Free PMC article.
-
Antagonistic Role of Aqueous Complexation in the Solvent Extraction and Separation of Rare Earth Ions.ACS Cent Sci. 2021 Nov 24;7(11):1908-1918. doi: 10.1021/acscentsci.1c00960. Epub 2021 Oct 25. ACS Cent Sci. 2021. PMID: 34841061 Free PMC article.
-
Stoichiometry of Lanthanide-Phosphate Complexes at the Water Surface Studied Using Vibrational Sum Frequency Generation Spectroscopy and DFT Calculations.J Phys Chem B. 2021 Jun 24;125(24):6727-6731. doi: 10.1021/acs.jpcb.1c04583. Epub 2021 Jun 14. J Phys Chem B. 2021. PMID: 34124914
-
Influence of the ionic liquid cation on the solvent extraction of trivalent rare-earth ions by mixtures of Cyanex 923 and ionic liquids.Dalton Trans. 2015 Jan 21;44(3):1379-87. doi: 10.1039/c4dt02766c. Dalton Trans. 2015. PMID: 25423581
-
Separation of rare earths from transition metals by liquid-liquid extraction from a molten salt hydrate to an ionic liquid phase.Dalton Trans. 2014 Feb 28;43(8):3186-95. doi: 10.1039/c3dt52541d. Dalton Trans. 2014. PMID: 24352299
Cited by
-
Thermodynamic Modeling of Salting Effects in Solvent Extraction of Cobalt(II) from Chloride Media by the Basic Extractant Methyltrioctylammonium Chloride.ACS Omega. 2021 Apr 20;6(17):11355-11366. doi: 10.1021/acsomega.1c00340. eCollection 2021 May 4. ACS Omega. 2021. PMID: 34056291 Free PMC article.
-
Molecular Dynamics Study of the Aggregation Behavior of N,N,N',N'-Tetraoctyl Diglycolamide.J Phys Chem B. 2022 Aug 25;126(33):6290-6300. doi: 10.1021/acs.jpcb.2c02198. Epub 2022 Aug 17. J Phys Chem B. 2022. PMID: 35975814 Free PMC article.
-
Phase transfer of metal cations by induced dynamic carrier agents: biphasic extraction based on dynamic covalent chemistry.Chem Sci. 2020 Oct 2;11(42):11468-11477. doi: 10.1039/d0sc04098c. Chem Sci. 2020. PMID: 34094390 Free PMC article.
-
Insights into coordination and ligand trends of lanthanide complexes from the Cambridge Structural Database.Sci Rep. 2024 May 17;14(1):11301. doi: 10.1038/s41598-024-62074-3. Sci Rep. 2024. PMID: 38760382 Free PMC article.
-
Hierarchical Aggregation in a Complex Fluid─The Role of Isomeric Interconversion.J Phys Chem B. 2023 Mar 9;127(9):2052-2065. doi: 10.1021/acs.jpcb.2c07527. Epub 2023 Feb 23. J Phys Chem B. 2023. PMID: 36821599 Free PMC article.
References
-
- Binnemans K.; Jones P. T.; Blanpain B.; Van Gerven T.; Yang Y.; Walton A.; Buchert M. Recycling of rare earths: A critical review. J. Cleaner Prod. 2013, 51, 1–22. 10.1016/j.jclepro.2012.12.037. - DOI
-
- Jha M. K.; Kumari A.; Panda R.; Kumar J. R.; Yoo K.; Lee J. Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. 10.1016/j.hydromet.2016.01.035. - DOI
-
- Du X.; Graedel T. E. Global rare earth in-use stocks in NdFeB permanent magnets. J. Ind. Ecol. 2011, 15, 836–843. 10.1111/j.1530-9290.2011.00362.x. - DOI
-
- Lumetta G. J.; Gelis A. V.; Vandegrift G. F. Review: Solvent Systems Combining Neutral and Acidic Extractants for Separating Trivalent Lanthanides from the Transuranic Elements. Solvent Extr. Ion Exch. 2010, 28, 287–312. 10.1080/07366291003684253. - DOI
-
- Gelis A. V.; Lumetta G. J. Actinide Lanthanide Separation Process-ALSEP. Ind. Eng. Chem. Res. 2014, 53, 1624–1631. 10.1021/ie403569e. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

