Opioid Tolerance in Critical Illness
- PMID: 30673555
- PMCID: PMC6897318
- DOI: 10.1056/NEJMra1800222
Opioid Tolerance in Critical Illness
Figures
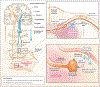


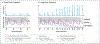
Comment in
-
Opioid Tolerance in Critical Illness.N Engl J Med. 2019 Apr 18;380(16):e26. doi: 10.1056/NEJMc1902646. N Engl J Med. 2019. PMID: 30995389 No abstract available.
References
-
- Cullen DJ, Sweitzer BJ, Bates DW, Burdick E, Edmondson A, Leape LL. Preventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care units. Crit Care Med 1997; 25: 1289–97. - PubMed
-
- Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41: 263–306. - PubMed
-
- Roeckel LA, Le Coz GM, Gavériaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience 2016; 338:1 60–82. - PubMed
-
- Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008; 24: 479–96. - PubMed
-
- Puntillo KA, Naidu R. Chronic pain disorders after critical illness and ICU-acquired opioid dependence: two clinical conundra. Curr Opin Crit Care 2016; 22: 506–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
