Relationship between senescence in macaques and bone marrow mesenchymal stem cells and the molecular mechanism
- PMID: 30673631
- PMCID: PMC6366955
- DOI: 10.18632/aging.101762
Relationship between senescence in macaques and bone marrow mesenchymal stem cells and the molecular mechanism
Abstract
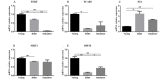
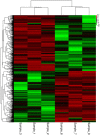
The relationship between bone marrow mesenchymal stem cells (BMSCs) and aging, as well as the antiaging effects of BMSCs, was observed. An aging macaque BMSC model was established. We isolated BMSCs from young and aged macaques and used RT-PCR and Western blot to confirm the aging-related mRNAs and their expression, revealing that TERT, SIRT1 and SIRT6 expression was decreased in the aged BMSCs. The morphology, immunophenotype, differentiation potential, proliferation potential, and antiaging effects of aged and young BMSCs on 293T cells were compared. The expression of aging-related genes and the difference between the secreted cytokines in natural aging and induced aging BMSCs were observed. The transcriptome of peripheral blood mononuclear cells from macaques was analyzed by high-throughput sequencing. Finally, the transcriptional characteristics and regulatory mechanisms of gene transcription in aging macaques were investigated.
Keywords: bone marrow mesenchymal stem cells; cytokines; macaque; senescence; transcriptome sequencing.
Conflict of interest statement
Figures












Similar articles
-
Multi-Parameter Analysis of Biobanked Human Bone Marrow Stromal Cells Shows Little Influence for Donor Age and Mild Comorbidities on Phenotypic and Functional Properties.Front Immunol. 2019 Nov 8;10:2474. doi: 10.3389/fimmu.2019.02474. eCollection 2019. Front Immunol. 2019. PMID: 31781089 Free PMC article.
-
Secretion of IL-6 and IL-8 in the senescence of bone marrow mesenchymal stem cells is regulated by autophagy via FoxO3a.Exp Gerontol. 2023 Feb;172:112062. doi: 10.1016/j.exger.2022.112062. Epub 2022 Dec 14. Exp Gerontol. 2023. PMID: 36526098
-
A pH probe inhibits senescence in mesenchymal stem cells.Stem Cell Res Ther. 2018 Dec 7;9(1):343. doi: 10.1186/s13287-018-1081-0. Stem Cell Res Ther. 2018. PMID: 30526663 Free PMC article.
-
Aging and lineage allocation changes of bone marrow skeletal (stromal) stem cells.Bone. 2019 Jun;123:265-273. doi: 10.1016/j.bone.2019.03.041. Epub 2019 Apr 1. Bone. 2019. PMID: 30946971 Review.
-
Characterization of bone marrow-derived mesenchymal stem cells in aging.Bone. 2015 Jan;70:37-47. doi: 10.1016/j.bone.2014.10.014. Epub 2014 Oct 28. Bone. 2015. PMID: 25445445 Review.
Cited by
-
Bone marrow mesenchymal stem cells derived from juvenile macaques reversed ovarian ageing in elderly macaques.Stem Cell Res Ther. 2021 Aug 18;12(1):460. doi: 10.1186/s13287-021-02486-4. Stem Cell Res Ther. 2021. PMID: 34407863 Free PMC article.
-
The effects of BMMSC treatment on lung tissue degeneration in elderly macaques.Stem Cell Res Ther. 2021 Mar 1;12(1):156. doi: 10.1186/s13287-021-02201-3. Stem Cell Res Ther. 2021. PMID: 33648583 Free PMC article.
-
The Safe and Efficacious Use of Secretome From Fibroblasts and Adipose-derived (but not Bone Marrow-derived) Mesenchymal Stem Cells for Skin Therapeutics.J Clin Aesthet Dermatol. 2019 Aug;12(8):E57-E69. Epub 2019 Aug 1. J Clin Aesthet Dermatol. 2019. PMID: 31531174 Free PMC article. Review.
-
Risk factors analysis and nomogram development for myelosuppression in diffuse large B-cell lymphoma patients undergoing first-line chemotherapy: a dual-centre retrospective cohort study.PeerJ. 2025 Jun 17;13:e19539. doi: 10.7717/peerj.19539. eCollection 2025. PeerJ. 2025. PMID: 40547311 Free PMC article.
-
In Vitro and In Vivo Modeling of Normal and Leukemic Bone Marrow Niches: Cellular Senescence Contribution to Leukemia Induction and Progression.Int J Mol Sci. 2022 Jul 1;23(13):7350. doi: 10.3390/ijms23137350. Int J Mol Sci. 2022. PMID: 35806354 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

