Stimuli Responsive Poly(Vinyl Caprolactam) Gels for Biomedical Applications
- PMID: 30674138
- PMCID: PMC6318617
- DOI: 10.3390/gels2010006
Stimuli Responsive Poly(Vinyl Caprolactam) Gels for Biomedical Applications
Abstract
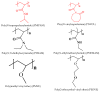
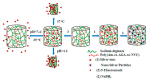
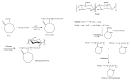
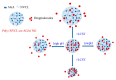
Poly(vinyl caprolactam) (PNVCL) is one of the most important thermoresponsive polymers because it is similar to poly(N-isopropyl acrylamide). PNVCL precipitates from aqueous solutions in a physiological temperature range (32⁻34 °C). The use of PNVCL instead of PNIPAM is considered advantageous because of the assumed lower toxicity of PNVCL. PNVCL copolymer gels are sensitive to external stimuli, such as temperature and pH; which gives them a wide range of biomedical applications and consequently attracts considerable scientific interest. This review focuses on the recent studies on PNVCL-based stimuli responsive three dimensional hydrogels (macro, micro, and nano) for biomedical applications. This review also covers the future outlooks of PNVCL-based gels for biomedical applications, particularly in the drug delivery field.
Keywords: biomedical applications; gels; poly(vinyl caprolactam); stimuli responsive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Langer R., Peppas N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49:2990–3006. doi: 10.1002/aic.690491202. - DOI
-
- Gil E.S., Hudson S.M. Stimuli responsive polymers and their conjugates. Prog. Polym. Sci. 2004;9:1173–1222. doi: 10.1016/j.progpolymsci.2004.08.003. - DOI
-
- Tanaka T. Collapse of gels and the critical end point. Phys. Rev. Lett. 1978;40:820–824. doi: 10.1103/PhysRevLett.40.820. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

