Magnetic Gel Composites for Hyperthermia Cancer Therapy
- PMID: 30674170
- PMCID: PMC6318599
- DOI: 10.3390/gels1020135
Magnetic Gel Composites for Hyperthermia Cancer Therapy
Abstract
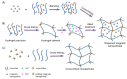
Hyperthermia therapy is a medical treatment based on the exposition of body tissue to slightly higher temperatures than physiological (i.e., between 41 and 46 °C) to damage and kill cancer cells or to make them more susceptible to the effects of radiation and anti-cancer drugs. Among several methods suitable for heating tumor areas, magnetic hyperthermia involves the introduction of magnetic micro/nanoparticles into the tumor tissue, followed by the application of an external magnetic field at fixed frequency and amplitude. A very interesting approach for magnetic hyperthermia is the use of biocompatible thermo-responsive magnetic gels made by the incorporation of the magnetic particles into cross-linked polymer gels. Mainly because of the hysteresis loss from the magnetic particles subjected to a magnetic field, the temperature of the system goes up and, once the temperature crosses the lower critical solution temperature, thermo-responsive gels undergo large volume changes and may deliver anti-cancer drug molecules that have been previously entrapped in their networks. This tutorial review describes the main properties and formulations of magnetic gel composites conceived for magnetic hyperthermia therapy.
Keywords: cancer therapy; composites; drug delivery; hydrogel; hyperthermia; magnetic nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Optimization Study on Specific Loss Power in Superparamagnetic Hyperthermia with Magnetite Nanoparticles for High Efficiency in Alternative Cancer Therapy.Nanomaterials (Basel). 2020 Dec 26;11(1):40. doi: 10.3390/nano11010040. Nanomaterials (Basel). 2020. PMID: 33375292 Free PMC article.
-
Magnetically responsive peptide coacervates for dual hyperthermia and chemotherapy treatments of liver cancer.Acta Biomater. 2020 Jul 1;110:221-230. doi: 10.1016/j.actbio.2020.04.024. Epub 2020 May 15. Acta Biomater. 2020. PMID: 32422317
-
Magnetic cryogels as a shape-selective and customizable platform for hyperthermia-mediated drug delivery.Sci Rep. 2022 Jun 10;12(1):9654. doi: 10.1038/s41598-022-13572-9. Sci Rep. 2022. PMID: 35688935 Free PMC article.
-
How Magnetic Composites are Effective Anticancer Therapeutics? A Comprehensive Review of the Literature.Int J Nanomedicine. 2023 Jun 30;18:3535-3575. doi: 10.2147/IJN.S375964. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37409027 Free PMC article. Review.
-
Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy.Nanomaterials (Basel). 2021 May 1;11(5):1203. doi: 10.3390/nano11051203. Nanomaterials (Basel). 2021. PMID: 34062851 Free PMC article. Review.
Cited by
-
Gold-Nanoparticle Hybrid Nanostructures for Multimodal Cancer Therapy.Nanomaterials (Basel). 2022 Oct 21;12(20):3706. doi: 10.3390/nano12203706. Nanomaterials (Basel). 2022. PMID: 36296896 Free PMC article. Review.
-
Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water.Gels. 2023 Aug 1;9(8):621. doi: 10.3390/gels9080621. Gels. 2023. PMID: 37623076 Free PMC article.
-
Nanoformulation Design Including MamC-Mediated Biomimetic Nanoparticles Allows the Simultaneous Application of Targeted Drug Delivery and Magnetic Hyperthermia.Polymers (Basel). 2020 Aug 15;12(8):1832. doi: 10.3390/polym12081832. Polymers (Basel). 2020. PMID: 32824256 Free PMC article.
-
Magnetic Nanoparticle Composites: Synergistic Effects and Applications.Adv Sci (Weinh). 2021 May 5;8(12):2004951. doi: 10.1002/advs.202004951. eCollection 2021 Jun. Adv Sci (Weinh). 2021. PMID: 34194936 Free PMC article. Review.
-
Research Progress of Thermosensitive Hydrogel in Tumor Therapeutic.Nanoscale Res Lett. 2021 Mar 4;16(1):42. doi: 10.1186/s11671-021-03502-5. Nanoscale Res Lett. 2021. PMID: 33665739 Free PMC article. Review.
References
-
- Baronzio G.F. Hyperthermia in Cancer Treatment: A Primer. Springer; Berlin, Germany: 2006.
-
- Hildebrandt B., Wust P., Ahlers O., Dieing A., Sreenivasa G., Kerner T., Felix R., Riess H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002;43:33–56. - PubMed
-
- Freeman C., Edward C.H., Brady L.W., Wazer D.E. Perez and Brady’s Principles and Practice of Radiation Oncology. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2008. pp. 637–644.
-
- Sneed P.K., Stauffer P.R., McDermott M.W., Diederich C.J., Lamborn K.R., Prados M.D., Chang S., Weaver K.A., Spry L., Malec M.K., et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 1998;40:287–295. doi: 10.1016/S0360-3016(97)00731-1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

