The phenylketonuria-associated substitution R68S converts phenylalanine hydroxylase to a constitutively active enzyme but reduces its stability
- PMID: 30674554
- PMCID: PMC6433070
- DOI: 10.1074/jbc.RA118.006477
The phenylketonuria-associated substitution R68S converts phenylalanine hydroxylase to a constitutively active enzyme but reduces its stability
Abstract
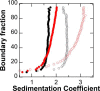
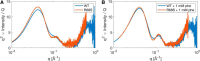
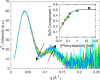
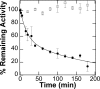
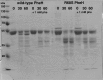
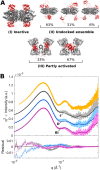
The naturally occurring R68S substitution of phenylalanine hydroxylase (PheH) causes phenylketonuria (PKU). However, the molecular basis for how the R68S variant leads to PKU remains unclear. Kinetic characterization of R68S PheH establishes that the enzyme is fully active in the absence of allosteric binding of phenylalanine, in contrast to the WT enzyme. Analytical ultracentrifugation establishes that the isolated regulatory domain of R68S PheH is predominantly monomeric in the absence of phenylalanine and dimerizes in its presence, similar to the regulatory domain of the WT enzyme. Fluorescence and small-angle X-ray scattering analyses establish that the overall conformation of the resting form of R68S PheH is different from that of the WT enzyme. The data are consistent with the substitution disrupting the interface between the catalytic and regulatory domains of the enzyme, shifting the equilibrium between the resting and activated forms ∼200-fold, so that the resting form of R68S PheH is ∼70% in the activated conformation. However, R68S PheH loses activity 2 orders of magnitude more rapidly than the WT enzyme at 37 °C and is significantly more sensitive to proteolysis. We propose that, even though this substitution converts the enzyme to a constitutively active enzyme, it results in PKU because of the decrease in protein stability.
Keywords: allosteric regulation; allostery; analytical ultracentrifugation; enzyme kinetics; enzyme stability; hydroxylase; phenylalanine hydroxylase; phenylketonuria; protein conformation; protein structure; small-angle X-ray scattering (SAXS).
© 2019 Khan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


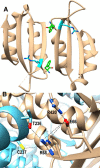
References
-
- Shiman R., Jones S. H., and Gray D. W. (1990) Mechanism of phenylalanine regulation of phenylalanine hydroxylase. J. Biol. Chem. 265, 11633–11642 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

