Bifidobacterium Abundance in Early Infancy and Vaccine Response at 2 Years of Age
- PMID: 30674610
- PMCID: PMC6361348
- DOI: 10.1542/peds.2018-1489
Bifidobacterium Abundance in Early Infancy and Vaccine Response at 2 Years of Age
Abstract
Background: The intestinal microbiome in early infancy affects immunologic development and thus may affect vaccine memory, though few prospective studies have examined such associations. We examined the association of Bifidobacterium levels in early infancy with memory responses to early vaccination measured at 2 years of age.
Methods: In this prospective observational study, we examined the association of Bifidobacterium abundance in the stool of healthy infants at 6 to 15 weeks of age, near the time of vaccination, with T-cell and antibody responses measured at 6 weeks, 15 weeks, and 2 years of age. Infants were vaccinated with Bacillus Calmette-Guérin (BCG) (at birth), oral polio virus (at birth and at 6, 10, and 14 weeks), tetanus toxoid (TT) (at 6, 10, and 14 weeks), and hepatitis B virus (at 6, 10, and 14 weeks). Fecal Bifidobacterium was measured at 6, 11, and 15 weeks. Bifidobacterium species and subspecies were measured at 6 weeks.
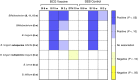
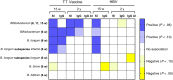
Results: Mean Bifidobacterium abundance in early infancy was positively associated with the CD4 T-cell responses to BCG, TT, and hepatitis B virus at 15 weeks, with CD4 responses to BCG and TT at 2 years, and with plasma TT-specific immunoglobulin G and stool polio-specific immunoglobulin A at 2 years. Similar associations were seen for the predominant subspecies, Bifidobacterium longum subspecies infantis.
Conclusions: Bifidobacterium abundance in early infancy may increase protective efficacy of vaccines by enhancing immunologic memory. This hypothesis could be tested in clinical trials of interventions to optimize Bifidobacterium abundance in appropriate populations.
Trial registration: ClinicalTrials.gov NCT01583972 NCT02027610.
Copyright © 2019 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Underwood’s institution (University of California, Davis) has received grant support from Evolve Biosystems to fund a clinical trial of a probiotic in term infants; he has consulted for Avexegen and received payment for travel and lectures from Abbott. Dr Mills is a cofounder of and consultant for Evolve Biosystems and has stock and stock options therein; he has received payment for lectures from Nestle and Abbott; the other authors have indicated they have no potential conflicts of interest to disclose.
Figures


References
-
- Ehreth J. The global value of vaccination. Vaccine. 2003;21(7-8):596–600 - PubMed
-
- Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of Tcell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines. 2004;3(1):23–34 - PubMed
-
- Siegrist C-A. Vaccine immunology In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 6th ed. Philadelphia, PA: Saunders; 2013:14–32
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

