Repurposing Pilocarpine Hydrochloride for Treatment of Candida albicans Infections
- PMID: 30674648
- PMCID: PMC6344604
- DOI: 10.1128/mSphere.00689-18
Repurposing Pilocarpine Hydrochloride for Treatment of Candida albicans Infections
Abstract
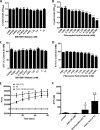
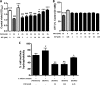
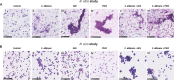
Acetylcholine modulates the virulence of Candidaalbicans and regulates an appropriate immune response to infection in a Galleria mellonella infection model. Indeed, the evidence suggests that C. albicans possesses a functional cholinergic receptor that can regulate filamentous growth and biofilm formation. Furthermore, G. mellonella immune cell subsets possess repertories of cholinergic receptors which regulate an effective and appropriate cellular immune response to C. albicans infection. This study aimed to investigate the cholinergic receptor subtype involved in regulation of filamentous growth and biofilm formation by C. albicans and determine the roles of cholinergic receptors in modulation of G. mellonella immune cell subsets. The general muscarinic receptor agonist, pilocarpine hydrochloride, inhibited C. albicans biofilm formation and pathogenicity, a phenomenon that could be reversed using the general muscarinic receptor antagonist, scopolamine. Pilocarpine hydrochloride protected G. mellonella larvae from C. albicans infection via inhibition of C. albicans filamentation and appropriate regulation of cellular immunity. However, scopolamine abrogated the capacity of pilocarpine hydrochloride to protect G. mellonella larvae from C. albicans infection. Furthermore, acetylcholine and pilocarpine hydrochloride exhibited differential modulatory capabilities on Galleria mellonella hemocyte responses to C. albicans The data in this article demonstrate that a muscarinic receptor modulates C. albicans filamentation and biofilm formation. Furthermore, the results suggest that G. mellonella hemocyte subsets possess unique repertoires of cholinergic receptors that regulate their differentiation, activation, and function in contrasting manners. Therefore, targeting cholinergic receptors by repurposing currently licensed cholinergic drugs may offer novel therapeutic solutions for the prevention or treatment of fungal infections.IMPORTANCECandida albicans is the most common human fungal pathogen with an estimated crude mortality rate of 40%. The ability of the organism to switch from the yeast to hyphal form and produce biofilms are important virulence factors. C. albicans infections are combatted by the host immune system. However, Candida triggers a strong inflammatory response that, if not appropriately regulated, can damage host tissues. Therefore, it is important that the host immune response eliminates the fungus but limits tissue damage. This study provides evidence that targeting cholinergic receptors cannot only curb the virulence of C. albicans by inhibiting filamentous growth and biofilm formation but can also appropriately regulate the host immune response to induce rapid clearance with limited damage to vital tissues. This article provides evidence that repurposing licensed drugs that target cholinergic receptors may offer novel therapeutic solutions for the prevention or treatment of fungal infections.
Keywords: Candida albicans; Galleria mellonella; biofilm; muscarinic; pilocarpine hydrochloride; repurposing.
Copyright © 2019 Nile et al.
Figures


References
-
- Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ. 2003. The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci 72:2055–2061. - PubMed
-
- Kawashima K, Fujii T. 2008. Basic and clinical aspects of non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance. J Pharmacol Sci 106:167–173. - PubMed
-
- Fernandez-Cabezudo MJ, Lorke DE, Azimullah S, Mechkarska M, Hasan MY, Petroianu GA, Al-Ramadi BK. 2010. Cholinergic stimulation of the immune system protects against lethal infection by Salmonella enterica serovar Typhimurium. Immunology 130:388–398. doi:10.1111/j.1365-2567.2009.03238.x. - DOI - PMC - PubMed
