Interrelationships between structure and function during the hemostatic response to injury
- PMID: 30674670
- PMCID: PMC6369752
- DOI: 10.1073/pnas.1813642116
Interrelationships between structure and function during the hemostatic response to injury
Abstract
Extensive studies have detailed the molecular regulation of individual components of the hemostatic system, including platelets, coagulation factors, and regulatory proteins. Questions remain, however, about how these elements are integrated at the systems level within a rapidly changing physical environment. To answer some of these questions, we developed a puncture injury model in mouse jugular veins that combines high-resolution, multimodal imaging with functional readouts in vivo. The results reveal striking spatial regulation of platelet activation and fibrin formation that could not be inferred from studies performed ex vivo. As in the microcirculation, where previous studies have been performed, gradients of platelet activation are readily apparent, as is an asymmetrical distribution of fibrin deposition and thrombin activity. Both are oriented from the outer to the inner surface of the damaged vessel wall, with a greater extent of platelet activation and fibrin accumulation on the outside than the inside. Further, we show that the importance of P2Y12 signaling in establishing a competent hemostatic plug is related to the size of the injury, thus limiting its contribution to hemostasis to specific physiologic contexts. Taken together, these studies offer insights into the organization of hemostatic plugs, provide a detailed understanding of the adverse bleeding associated with a widely prescribed class of antiplatelet agents, and highlight differences between hemostasis and thrombosis that may suggest alternative therapeutic approaches.
Keywords: P2Y12; coagulation; hemostasis; platelets; thrombin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

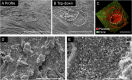
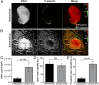



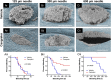

References
-
- Silvain J, et al. Thrombus composition in sudden cardiac death from acute myocardial infarction. Resuscitation. 2017;113:108–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

