Small molecule ISRIB suppresses the integrated stress response within a defined window of activation
- PMID: 30674674
- PMCID: PMC6369741
- DOI: 10.1073/pnas.1815767116
Small molecule ISRIB suppresses the integrated stress response within a defined window of activation
Erratum in
-
Correction to Supporting Information for Rabouw et al., Small molecule ISRIB suppresses the integrated stress response within a defined window of activation.Proc Natl Acad Sci U S A. 2021 Apr 27;118(17):e2103599118. doi: 10.1073/pnas.2103599118. Proc Natl Acad Sci U S A. 2021. PMID: 33875607 Free PMC article. No abstract available.
Abstract
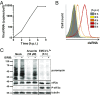
Activation of the integrated stress response (ISR) by a variety of stresses triggers phosphorylation of the α-subunit of translation initiation factor eIF2. P-eIF2α inhibits eIF2B, the guanine nucleotide exchange factor that recycles inactive eIF2•GDP to active eIF2•GTP. eIF2 phosphorylation thereby represses translation. Persistent activation of the ISR has been linked to the development of several neurological disorders, and modulation of the ISR promises new therapeutic strategies. Recently, a small-molecule ISR inhibitor (ISRIB) was identified that rescues translation in the presence of P-eIF2α by facilitating the assembly of more active eIF2B. ISRIB enhances cognitive memory processes and has therapeutic effects in brain-injured mice without displaying overt side effects. While using ISRIB to investigate the ISR in picornavirus-infected cells, we observed that ISRIB rescued translation early in infection when P-eIF2α levels were low, but not late in infection when P-eIF2α levels were high. By treating cells with varying concentrations of poly(I:C) or arsenite to induce the ISR, we provide additional proof that ISRIB is unable to inhibit the ISR when intracellular P-eIF2α concentrations exceed a critical threshold level. Together, our data demonstrate that the effects of pharmacological activation of eIF2B are tuned by P-eIF2α concentration. Thus, ISRIB can mitigate undesirable outcomes of low-level ISR activation that may manifest neurological disease but leaves the cytoprotective effects of acute ISR activation intact. The insensitivity of cells to ISRIB during acute ISR may explain why ISRIB does not cause overt toxic side effects in vivo.
Keywords: ISRIB; P-eIF2; eIF2B; integrated stress response.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: P.W. [University of California, San Francisco (UCSF) employee] currently holds ISRIB-related patents. These patents are licensed by UCSF to Genentech and Calico.
Figures
References
-
- Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol. 2008;10:1324–1332. - PubMed
-
- Takahara T, Maeda T. Transient sequestration of TORC1 into stress granules during heat stress. Mol Cell. 2012;47:242–252. - PubMed
-
- Pain VM. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747–771. - PubMed

