Interaction and Regulation Between Lipid Mediator Phosphatidic Acid and Circadian Clock Regulators
- PMID: 30674693
- PMCID: PMC6447011
- DOI: 10.1105/tpc.18.00675
Interaction and Regulation Between Lipid Mediator Phosphatidic Acid and Circadian Clock Regulators
Abstract
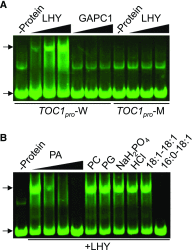
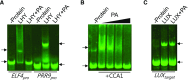
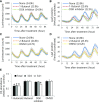
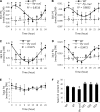
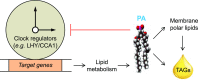
Circadian clocks play important roles in regulating cellular metabolism, but the reciprocal effect that metabolism has on the clock is largely unknown in plants. Here, we show that the central glycerolipid metabolite and lipid mediator phosphatidic acid (PA) interacts with and modulates the function of the core clock regulators LATE ELONGATED HYPOCOTYL (LHY) and CIRCADIAN CLOCK ASSOCIATED1 (CCA1) in Arabidopsis (Arabidopsis thaliana). PA reduced the ability of LHY and CCA1 to bind the promoter of their target gene TIMING OF CAB EXPRESSION1 Increased PA accumulation and inhibition of PA-producing enzymes had opposite effects on circadian clock outputs. Diurnal change in levels of several membrane phospholipid species, including PA, observed in wild type was lost in the LHY and CCA1 double knockout mutant. Storage lipid accumulation was also affected in the clock mutants. These results indicate that the interaction of PA with the clock regulator may function as a cellular conduit to integrate the circadian clock with lipid metabolism.
© 2019 American Society of Plant Biologists. All rights reserved.
Figures




Similar articles
-
LATE ELONGATED HYPOCOTYL regulates photoperiodic flowering via the circadian clock in Arabidopsis.BMC Plant Biol. 2016 May 20;16(1):114. doi: 10.1186/s12870-016-0810-8. BMC Plant Biol. 2016. PMID: 27207270 Free PMC article.
-
CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis.Plant Physiol. 2009 Jun;150(2):834-43. doi: 10.1104/pp.108.133272. Epub 2009 Feb 13. Plant Physiol. 2009. PMID: 19218364 Free PMC article.
-
The central circadian clock proteins CCA1 and LHY regulate iron homeostasis in Arabidopsis.J Integr Plant Biol. 2019 Feb;61(2):168-181. doi: 10.1111/jipb.12696. Epub 2018 Aug 31. J Integr Plant Biol. 2019. PMID: 29989313
-
Wheels within wheels: the plant circadian system.Trends Plant Sci. 2014 Apr;19(4):240-9. doi: 10.1016/j.tplants.2013.11.007. Epub 2013 Dec 24. Trends Plant Sci. 2014. PMID: 24373845 Free PMC article. Review.
-
Timing to grow: roles of clock in thermomorphogenesis.Trends Plant Sci. 2021 Dec;26(12):1248-1257. doi: 10.1016/j.tplants.2021.07.020. Epub 2021 Aug 14. Trends Plant Sci. 2021. PMID: 34404586 Review.
Cited by
-
Drought and heat stress mediated activation of lipid signaling in plants: a critical review.Front Plant Sci. 2023 Aug 10;14:1216835. doi: 10.3389/fpls.2023.1216835. eCollection 2023. Front Plant Sci. 2023. PMID: 37636093 Free PMC article. Review.
-
Monitoring lipid-protein interactions in planta using Förster resonance energy transfer.Methods Enzymol. 2023;683:243-252. doi: 10.1016/bs.mie.2022.08.040. Epub 2022 Nov 11. Methods Enzymol. 2023. PMID: 37087190 Free PMC article.
-
Engineering Fatty Acid Biosynthesis in Microalgae: Recent Progress and Perspectives.Mar Drugs. 2024 May 9;22(5):216. doi: 10.3390/md22050216. Mar Drugs. 2024. PMID: 38786607 Free PMC article. Review.
-
Phosphatidic acid signaling and function in nuclei.Prog Lipid Res. 2024 Jan;93:101267. doi: 10.1016/j.plipres.2023.101267. Epub 2023 Dec 26. Prog Lipid Res. 2024. PMID: 38154743 Free PMC article. Review.
-
Phosphatidic acid suppresses autophagy through competitive inhibition by binding GAPC (glyceraldehyde-3-phosphate dehydrogenase) and PGK (phosphoglycerate kinase) proteins.Autophagy. 2022 Nov;18(11):2656-2670. doi: 10.1080/15548627.2022.2046449. Epub 2022 Mar 15. Autophagy. 2022. PMID: 35289711 Free PMC article.
References
-
- Alabadí D., Oyama T., Yanovsky M.J., Harmon F.G., Más P., Kay S.A. (2001). Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293: 880–883. - PubMed
-
- Arisz S.A., Testerink C., Munnik T. (2009). Plant PA signaling via diacylglycerol kinase. Biochim. Biophys. Acta 1791: 869–875. - PubMed
-
- Asher G., Schibler U. (2011). Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 13: 125–137. - PubMed
-
- Atamian H.S., Creux N.M., Brown E.A., Garner A.G., Blackman B.K., Harmer S.L. (2016). Circadian regulation of sunflower heliotropism, floral orientation, and pollinator visits. Science 353: 587–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

