Carbon irradiation overcomes glioma radioresistance by eradicating stem cells and forming an antiangiogenic and immunopermissive niche
- PMID: 30674721
- PMCID: PMC6413779
- DOI: 10.1172/jci.insight.123837
Carbon irradiation overcomes glioma radioresistance by eradicating stem cells and forming an antiangiogenic and immunopermissive niche
Abstract
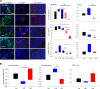
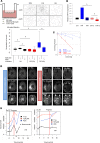
Tumor radioresistance leading to local therapy failure remains a major obstacle for successful treatment of high-grade glioma. We hypothesized that distinct radiobiological features of particle therapy with carbon ions may circumvent glioma radioresistance. We demonstrate that carbon irradiation (CIR) efficiently eradicates radioresistant patient-derived glioma stem cells (GSCs), leading to growth inhibition and prolonged survival. The impact of CIR at the tumor-stroma interface was further investigated in 2 syngeneic mouse and 2 orthotopic GSC xenograft models. Intriguingly, tumor regressions and long-term local controls were observed at doses greater than or equal to 15-Gy CIR. Fractionated CIR further prolonged survival. The enhanced relative biological effectiveness of CIR in vivo was attributed to its potent antiangiogenic effects and eradication of radioresistant hypoxic tumor cells. Blockade of the HIF1-α/stromal cell-derived factor 1/CXCR4 axis by CIR reduced the recruitment of microglia and myeloid-derived suppressor cells (CD11b+Gr1+). Consequently, CIR abrogated M2-like immune polarization and enhanced the influx of CD8+ cells, generating an immunopermissive niche. We report that radiotherapy with carbon ions could surmount several central glioma resistance mechanisms by eradicating hypoxic and stem cell-like tumor cells, as well as modulating the glioma niche toward an antiangiogenic and less immunosuppressive state. Conclusively, potentially novel rationales for CIR in conquering glioma radioresistance are provided.
Keywords: Brain cancer; Mouse models; Oncology; Therapeutics.
Conflict of interest statement
Figures




References
-
- Debus J, Abdollahi A. For the next trick: new discoveries in radiobiology applied to glioblastoma. Am Soc Clin Oncol Educ Book. 2014:e95–e99. - PubMed
-
- Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat. 2010;13(1–2):16–28. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

