Antiviral effects against influenza A virus infection by a short hairpin RNA targeting the non-coding terminal region of the viral nucleoprotein gene
- PMID: 30674743
- PMCID: PMC6451914
- DOI: 10.1292/jvms.18-0436
Antiviral effects against influenza A virus infection by a short hairpin RNA targeting the non-coding terminal region of the viral nucleoprotein gene
Abstract
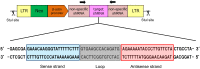
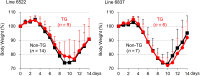
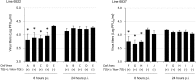
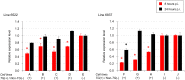
RNA interference (RNAi) can inhibit Influenza A virus (IAV) infection in a gene-specific manner. In this study, we constructed a transgene expressing a short hairpin RNA (shRNA) that targets the noncoding region of the IAV RNA gene encoding nucleoprotein (NP). To investigate the antiviral effects of the shRNA, we generated two transgenic mouse lines with this transgene. Unfortunately, there was no apparent difference in IAV resistance between transgenic and non-transgenic littermates. To further investigate the antiviral effects of the shRNA, we prepared mouse embryonic fibroblasts (MEFs) from transgenic and non-transgenic mice. In experimental infections using these MEFs, virus production of mouse-adapted IAV strain A/Puerto Rico/8/1934 (PR8) in the transgenic MEFs was suppressed by means of the down-regulation of the viral RNA gene transcription in the early stages of infection in comparison with non-transgenic MEFs. These results indicated that expression of the shRNA was able to confer antiviral properties against IAVs to MEFs, although the effects were limited. Our findings suggest that the shRNA targeting the noncoding region of the viral RNA (vRNA) of NP might be a supporting tool in developing influenza-resistant poultry.
Keywords: RNA interference; antiviral effect; influenza A virus; short hairpin RNA; transgenic mouse.
Figures
References
-
- Fujimoto Y., Tomioka Y., Takakuwa H., Uechi G., Yabuta T., Ozaki K., Suyama H., Yamamoto S., Morimatsu M., Mai Q., Yamashiro T., Ito T., Otsuki K., Ono E.2016. Cross-protective potential of anti-nucleoprotein human monoclonal antibodies against lethal influenza A virus infection. J. Gen. Virol. 97: 2104–2116. doi: 10.1099/jgv.0.000518 - DOI - PubMed
-
- Ge Q., McManus M. T., Nguyen T., Shen C. H., Sharp P. A., Eisen H. N., Chen J.2003. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc. Natl. Acad. Sci. U.S.A. 100: 2718–2723. doi: 10.1073/pnas.0437841100 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

