Recent Developments in Tough Hydrogels for Biomedical Applications
- PMID: 30674822
- PMCID: PMC6209285
- DOI: 10.3390/gels4020046
Recent Developments in Tough Hydrogels for Biomedical Applications
Abstract
A hydrogel is a three-dimensional polymer network with high water content and has been attractive for many biomedical applications due to its excellent biocompatibility. However, classic hydrogels are mechanically weak and unsuitable for most physiological load-bearing situations. Thus, the development of tough hydrogels used in the biomedical field becomes critical. This work reviews various strategies to fabricate tough hydrogels with the introduction of non-covalent bonds and the construction of stretchable polymer networks and interpenetrated networks, such as the so-called double-network hydrogel. Additionally, the design of tough hydrogels for tissue adhesive, tissue engineering, and soft actuators is reviewed.
Keywords: biomedical applications; soft actuators; tissue adhesives; tissue engineering; tough hydrogels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

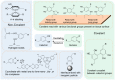
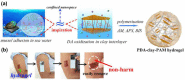
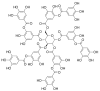


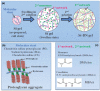


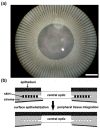
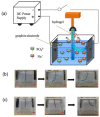

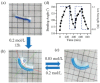

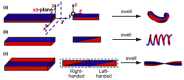
References
-
- Nguyen Q.V., Park J.H., Lee D.S. Injectable polymeric hydrogels for the delivery of therapeutic agents: A review. Eur. Polym. J. 2015;72:602–619. doi: 10.1016/j.eurpolymj.2015.03.016. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

