Viscoelastic Oxidized Alginates with Reversible Imine Type Crosslinks: Self-Healing, Injectable, and Bioprintable Hydrogels
- PMID: 30674861
- PMCID: PMC6318581
- DOI: 10.3390/gels4040085
Viscoelastic Oxidized Alginates with Reversible Imine Type Crosslinks: Self-Healing, Injectable, and Bioprintable Hydrogels
Abstract
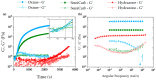
Bioprinting techniques allow for the recreation of 3D tissue-like structures. By deposition of hydrogels combined with cells (bioinks) in a spatially controlled way, one can create complex and multiscale structures. Despite this promise, the ability to deposit customizable cell-laden structures for soft tissues is still limited. Traditionally, bioprinting relies on hydrogels comprised of covalent or mostly static crosslinks. Yet, soft tissues and the extracellular matrix (ECM) possess viscoelastic properties, which can be more appropriately mimicked with hydrogels containing reversible crosslinks. In this study, we have investigated aldehyde containing oxidized alginate (ox-alg), combined with different cross-linkers, to develop a small library of viscoelastic, self-healing, and bioprintable hydrogels. By using distinctly different imine-type dynamic covalent chemistries (DCvC), (oxime, semicarbazone, and hydrazone), rational tuning of rheological and mechanical properties was possible. While all materials showed biocompatibility, we observed that the nature of imine type crosslink had a marked influence on hydrogel stiffness, viscoelasticity, self-healing, cell morphology, and printability. The semicarbazone and hydrazone crosslinks were found to be viscoelastic, self-healing, and printable-without the need for additional Ca2+ crosslinking-while also promoting the adhesion and spreading of fibroblasts. In contrast, the oxime cross-linked gels were found to be mostly elastic and showed neither self-healing, suitable printability, nor fibroblast spreading. The semicarbazone and hydrazone gels hold great potential as dynamic 3D cell culture systems, for therapeutics and cell delivery, and a newer generation of smart bioinks.
Keywords: bioprinting (BP); dynamic covalent chemistry (DCvC); dynamic hydrogel; hydrazone; oxidized alginate (ox-alg); oxime; reversible bonds; semicarbazone; tissue engineering; viscoelastic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bose S., Vahabzadeh S., Bandyopadhyay A., Keck W.M. Bone tissue engineering using 3D printing. Biochem. Pharmacol. 2013;16:496–504. doi: 10.1016/j.mattod.2013.11.017. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

