Toxicity and possible mechanisms of action of honokiol from Magnolia denudata seeds against four mosquito species
- PMID: 30674912
- PMCID: PMC6344527
- DOI: 10.1038/s41598-018-36558-y
Toxicity and possible mechanisms of action of honokiol from Magnolia denudata seeds against four mosquito species
Abstract
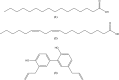
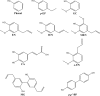
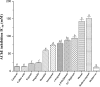
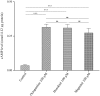
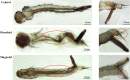
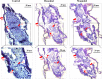
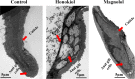
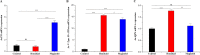
This study was performed to determine the toxicity and possible mechanism of the larvicidal action of honokiol, extracted from Magnolia denudata seeds, and its 10 related compounds against third-instar larvae of insecticide-susceptible Culex pipiens pallens, Aedes aegypti, and Aedes albopictus and Anopheles sinensis resistant to deltamethrin and temephos. Honokiol (LC50, 6.13-7.37 mg/L) was highly effective against larvae of all of the four mosquito species, although the toxicity of the compound was lower than that of the synthetic larvicide temephos. Structure-activity relationship analyses indicated that electron donor and/or bulky groups at the ortho or para positions of the phenol were required for toxicity. Honokiol moderately inhibited acetylcholinesterase and caused a considerable increase in cyclic AMP levels, indicating that it might act on both acetylcholinesterase and octopaminergic receptors. Microscopy analysis clearly indicated that honokiol was mainly targeted to the midgut epithelium and anal gills, resulting in variably dramatic degenerative responses of the midgut through sequential epithelial disorganization. Honokiol did not affect the AeCS1 mRNA expression level in Ae. aegypti larvae, but did enhance expression of the genes encoding vacuolar-type H+-ATPase and aquaporin 4, indicating that it may disturb the Na+, Cl- and K+ co-transport systems. These results demonstrate that honokiol merits further study as a potential larvicide, with a specific target site, and as a lead molecule for the control of mosquito populations.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Larvicidal activity of Magnolia denudata seed hydrodistillate constituents and related compounds and liquid formulations towards two susceptible and two wild mosquito species.Pest Manag Sci. 2016 May;72(5):897-906. doi: 10.1002/ps.4064. Epub 2015 Jul 16. Pest Manag Sci. 2016. PMID: 26085316
-
Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species.Parasit Vectors. 2015 Apr 19;8:237. doi: 10.1186/s13071-015-0848-8. Parasit Vectors. 2015. PMID: 25928224 Free PMC article.
-
Larvicidal activity of lignans and alkaloid identified in Zanthoxylum piperitum bark toward insecticide-susceptible and wild Culex pipiens pallens and Aedes aegypti.Parasit Vectors. 2017 May 4;10(1):221. doi: 10.1186/s13071-017-2154-0. Parasit Vectors. 2017. PMID: 28472971 Free PMC article.
-
Neuroprotective effects of honokiol: from chemistry to medicine.Biofactors. 2017 Nov;43(6):760-769. doi: 10.1002/biof.1385. Epub 2017 Aug 17. Biofactors. 2017. PMID: 28817221 Review.
-
Honokiol analogs: a novel class of anticancer agents targeting cell signaling pathways and other bioactivities.Future Med Chem. 2013 May;5(7):809-29. doi: 10.4155/fmc.13.32. Future Med Chem. 2013. PMID: 23651094 Review.
Cited by
-
Larvicidal Activity of Essential Oils From Piper Species Against Strains of Aedes aegypti (Diptera: Culicidae) Resistant to Pyrethroids.Front Plant Sci. 2021 Jun 4;12:685864. doi: 10.3389/fpls.2021.685864. eCollection 2021. Front Plant Sci. 2021. PMID: 34149785 Free PMC article.
-
Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties.Pharmaceutics. 2021 Feb 6;13(2):224. doi: 10.3390/pharmaceutics13020224. Pharmaceutics. 2021. PMID: 33561940 Free PMC article.
-
Impregnation of pectin-cedarwood essential oil nanocapsules onto mini cotton bag improves larvicidal performances.Sci Rep. 2020 Aug 24;10(1):14107. doi: 10.1038/s41598-020-70889-z. Sci Rep. 2020. PMID: 32839484 Free PMC article.
-
The Potential of Magnolia spp. in the Production of Alternative Pest Control Substances.Molecules. 2023 Jun 9;28(12):4681. doi: 10.3390/molecules28124681. Molecules. 2023. PMID: 37375236 Free PMC article. Review.
References
-
- Pages N, et al. Scientific review on mosquitoes and mosquito-borne disease. EFSA Supporting Publications. 2009;6:1–96. doi: 10.2903/sp.efsa.2009.EN-7. - DOI
-
- Confalonieri, U. et al. Human health in Climate Change 2007: Impacts, Adaptation and Vulnerability (eds Parry, M. L. et al.) 391–431 (Cambridge University Press, 2007).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

