Mechanisms and immunogenicity of nsPEF-induced cell death in B16F10 melanoma tumors
- PMID: 30674926
- PMCID: PMC6344591
- DOI: 10.1038/s41598-018-36527-5
Mechanisms and immunogenicity of nsPEF-induced cell death in B16F10 melanoma tumors
Abstract
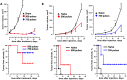
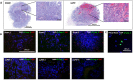
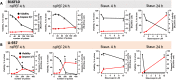
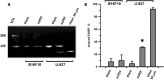
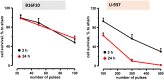
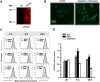
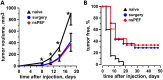
Accumulating data indicates that some cancer treatments can restore anticancer immunosurveillance through the induction of tumor immunogenic cell death (ICD). Nanosecond pulsed electric fields (nsPEF) have been shown to efficiently ablate melanoma tumors. In this study we investigated the mechanisms and immunogenicity of nsPEF-induced cell death in B16F10 melanoma tumors. Our data show that in vitro nsPEF (20-200, 200-ns pulses, 7 kV/cm, 2 Hz) caused a rapid dose-dependent cell death which was not accompanied by caspase activation or PARP cleavage. The lack of nsPEF-induced apoptosis was confirmed in vivo in B16F10 tumors. NsPEF also failed to trigger ICD-linked responses such as necroptosis and autophagy. Our results point at necrosis as the primary mechanism of cell death induced by nsPEF in B16F10 cells. We finally compared the antitumor immunity in animals treated with nsPEF (750, 200-ns, 25 kV/cm, 2 Hz) with animals were tumors were surgically removed. Compared to the naïve group where all animals developed tumors, nsPEF and surgery protected 33% (6/18) and 28.6% (4/14) of the animals, respectively. Our data suggest that, under our experimental conditions, the local ablation by nsPEF restored but did not boost the natural antitumor immunity which stays dormant in the tumor-bearing host.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

