Pro-resolving lipid mediator ameliorates obesity induced osteoarthritis by regulating synovial macrophage polarisation
- PMID: 30674985
- PMCID: PMC6344566
- DOI: 10.1038/s41598-018-36909-9
Pro-resolving lipid mediator ameliorates obesity induced osteoarthritis by regulating synovial macrophage polarisation
Abstract
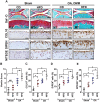
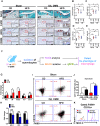
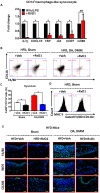
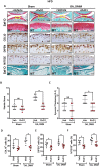
Non-resolved persistent macrophage-mediated synovial inflammation is considered as one of the main drivers of both the establishment and progression of obesity-associated osteoarthritis (OA). Herein, we used clodronate-loaded liposomes (CL) to locally deplete macrophages in the synovial joints to examine the role of macrophages in the progression of obesity-induced OA. Furthermore, resolvin D1 (RvD1), a unique family of pro-resolving lipid mediator derived from the omega-3 polyunsaturated fatty acid, have shown marked potency in changing the pro-inflammatory behaviour of the macrophages. We sought to determine whether RvD1 administration ameliorates obesity-induced OA by resolving macrophage-mediated synovitis. Therapeutic properties of RvD1 and macrophage depletion (CL) were tested for its ability to slow post-traumatic OA (PTOA) in obese mice models. PTOA was induced in C57Bl/6 mice fed with high-fat diet (HFD) by surgically destabilising the meniscus. Firstly, CL treatment showed beneficial effects in reducing synovitis and cartilage destruction in obese mice with PTOA. In vitro treatment with RvD1 decreased the levels of pro-inflammatory markers in CD14+ human macrophages. Furthermore, intra-articular treatment with RvD1 diminishes the progression of OA in the knee joint from mice as follows: (a) decreases macrophages infiltration in synovium, (b) reduces the number of pro-inflammatory macrophages in synovium and (c) improves the severity of synovitis and cartilage degradation. Thus, our results provide new evidence for the potential targeting of macrophages in the treatment of obesity-induced OA.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

