Efficient Nonviral Transfection of Human Bone Marrow Mesenchymal Stromal Cells Shown Using Placental Growth Factor Overexpression
- PMID: 30675166
- PMCID: PMC6323439
- DOI: 10.1155/2018/1310904
Efficient Nonviral Transfection of Human Bone Marrow Mesenchymal Stromal Cells Shown Using Placental Growth Factor Overexpression
Abstract
Background: Human mesenchymal stromal/stem cells (hMSCs) hold great therapeutic potential due to their immunomodulatory and tissue regenerative properties. Enhancement of biological features of hMSCs by transfection has become a focus of investigation for cell- and gene-based therapies. However, many of the current transient transfection methods result in either low transfection efficiency or high cytotoxicity.
Methods: In order to find a transfection method that would address the current issues of low transfection efficiency and high cytotoxicity, 6 commercially available cationic lipid and polymer reagents were tested on human bone marrow-derived MSCs (hBM-MSCs) using GFP as a reporter gene. One transfection method using TransIT-2020 was selected and tested with an emphasis on cell quality (viability, identity, and yield), as well as efficacy with a human placental growth factor (PlGF) plasmid.
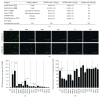
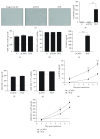
Results: TransIT-2020 yielded the highest fluorescence signal per cell out of the methods that did not decrease cell recovery. Transfecting GFP to 5 hBM-MSC donors using TransIT-2020 yielded 24-36% GFP-expressing cells with a viability of 85-96%. hBM-MSC identity was unaffected as CD90, CD105, and CD73 markers were retained (>95%+) after transfection. When this method was applied to PlGF expression, there was up to a 220-fold increase in secretion. Both growth and secretion of PlGF in overexpressing hBM-MSC were sustained over 7 days, confirming the sustainability and applicability of the TransIT-2020 transfection system.
Discussion: We report a simple and efficient method for transient transfection that has not been reported for hBM-MSCs, encompassing high levels of plasmid expression without significant changes to fundamental hBM-MSC characteristics.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

