Radium-223 in patients with metastatic castration-resistant prostate cancer: Efficacy and safety in clinical practice
- PMID: 30675201
- PMCID: PMC6341517
- DOI: 10.3892/ol.2018.9785
Radium-223 in patients with metastatic castration-resistant prostate cancer: Efficacy and safety in clinical practice
Abstract
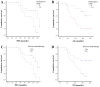
Radium-223 has improved overall survival (OS) and reduced symptomatic skeletal events (SSE) in patients with metastatic castration-resistant prostate cancer (mCRPC) and bone metastases (ALSYMPCA trial). Our aim was to assess clinical and biochemical factors related to survival, safety and survival outcomes of Radium-223 in a clinical practice setting. We retrospectively analysed 32 mCRPC patients treated with Radium-223, assessing bone scan, pain reduction, alkaline phosphatase (ALP) and prostate-specific antigen (PSA) response (≥30% reduction). At scintigraphic assessment, 41% had partial response with a disease control rate of 91%; 56% had ALP response and 25% had PSA response; 41% had pain reduction with pain control of 72%. Scintigraphic response and stability were correlated with longer median progression-free survival (mPFS) (13 and 12 vs. 6 months; P=0.002) and mOS (16 and 12 vs. 6 months; P=0.003). ALP response was associated with longer mPFS (13 vs. 12 months; P=0.2) and mOS (16 vs. 12 months; P=0.2). PSA response was associated with longer mPFS (13 vs. 12 months; P=0.02), whereas mOS could not be computed. Pain response and stability were associated with survival benefit according to mPFS (13 and 12 vs. 9 months) and mOS (both 16 vs. 12 months) without statistical significance. Baseline ALP <220 UI/l, Eastern Cooperative Oncology Group (ECOG) performance status 0 and absence of previous chemotherapy correlated with statistically significantly longer survival outcomes. Skeletal-related events (SRE) occurred in three patients and median time to first SRE was 9.5 months, mPFS was 12 months and mOS 14 months. G3-G4 toxicities developed in 16% of patients. Our results are in line with those reported in the pivotal trial and in other retrospective studies. In conclusion, Radium-223 was associated with high scintigraphic, biochemical and pain response rates and was tolerated well by most patients. Response to Radium-223 and better baseline factors correlated to longer survival in clinical practice experience as in the clinical trial setting.
Keywords: alkaline phosphatase; bone scan; metastatic castration-resistant prostate cancer; pain; prostate-specific antigen; radium-223.
Figures



References
-
- Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, Lennernäs B, Petersson U, Johannessen DC, Sokal M, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. - DOI - PubMed
-
- Nilsson S, Strang P, Aksnes AK, Franzèn L, Olivier P, Pecking A, Staffurth J, Vasanthan S, Andersson C, Bruland ØS. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer. 2012;48:678–686. doi: 10.1016/j.ejca.2011.12.023. - DOI - PubMed
-
- Parker CC, Pascoe S, Chodacki A, O'Sullivan JM, Germá JR, O'Bryan-Tear CG, Haider T, Hoskin P. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol. 2013;63:189–197. doi: 10.1016/j.eururo.2012.09.008. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
