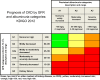
KDIGO 2018 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease
- PMID: 30675443
- PMCID: PMC6336217
- DOI: 10.1016/j.kisu.2018.06.001
KDIGO 2018 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease
Figures






















Comment in
-
Response to the KDOQI US Commentary on the 2018 KDIGO Hepatitis C Guideline.Am J Kidney Dis. 2021 Jan;77(1):152. doi: 10.1053/j.ajkd.2020.07.014. Epub 2020 Sep 3. Am J Kidney Dis. 2021. PMID: 32891626 No abstract available.
-
In Reply to 'Response to the KDOQI US Commentary on the 2018 KDIGO Hepatitis C Guideline'.Am J Kidney Dis. 2021 Jan;77(1):152-153. doi: 10.1053/j.ajkd.2020.08.001. Epub 2020 Sep 3. Am J Kidney Dis. 2021. PMID: 32891628 No abstract available.
References
-
- The Lancet Gastroenterology Hepatology Access to HCV treatments: hurdles not barriers. Lancet Gastroenterol Hepatol. 2017;2:1. - PubMed
-
- World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. July 2018. Available at: http://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/. Accessed July 27, 2018. - PubMed
-
- Bergman S., Accortt N., Turner A. Hepatitis C infection is acquired pre-ESRD. Am J Kidney Dis. 2005;45:684–689. - PubMed
LinkOut - more resources
Full Text Sources
Medical

