MEP50/PRMT5-mediated methylation activates GLI1 in Hedgehog signalling through inhibition of ubiquitination by the ITCH/NUMB complex
- PMID: 30675521
- PMCID: PMC6338668
- DOI: 10.1038/s42003-018-0275-4
MEP50/PRMT5-mediated methylation activates GLI1 in Hedgehog signalling through inhibition of ubiquitination by the ITCH/NUMB complex
Abstract
Transcription factor GLI1 is an effecter of Hedgehog (HH) signalling and activated in a broad spectrum of cancers. However, the role of the HH-GLI1 pathway in cancer and the activation mechanism of GLI1 in HH signalling after dissociation from its inhibitor, SUFU, are not fully understood. Here, we found that GLI1 associated with the methylosome protein 50 (MEP50)/protein arginine methyltransferase 5 (PRMT5) complex and was methylated. Association of MEP50/PRMT5 with GLI1 was enhanced and expression of MEP50 and PRMT5 was activated by HH signals, suggesting their role in positive feedback regulation. Methylated GLI1 lost its ability to bind ubiquitin ligase ITCH/NUMB, resulting in nuclear accumulation and activation of GLI1. Moreover, protein expression of GLI1 was enhanced by MEP50/PRMT5 and expression of MEP50, PRMT5, and GLI1 target genes was upregulated in HH-expressing cancers. These results suggest that MEP50/PRMT5 is important for HH signal-induced GLI1 activation, especially in cancers.
Conflict of interest statement
The authors declare no competing interests.
Figures

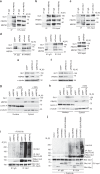
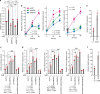


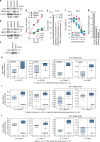
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

