Clinical Characteristics and Electrophysiological Mechanisms Underlying Brugada ECG in Patients With Severe Hyperkalemia
- PMID: 30675825
- PMCID: PMC6405573
- DOI: 10.1161/JAHA.118.010115
Clinical Characteristics and Electrophysiological Mechanisms Underlying Brugada ECG in Patients With Severe Hyperkalemia
Abstract
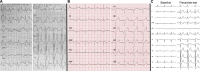
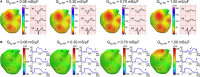
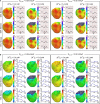
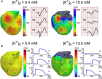
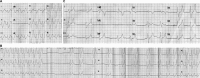
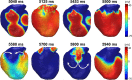
Background Several metabolic conditions can cause the Brugada ECG pattern, also called Brugada phenotype (BrPh). We aimed to define the clinical characteristics and outcome of BrPh patients and elucidate the mechanisms underlying BrPh attributed to hyperkalemia. Methods and Results We prospectively identified patients hospitalized with severe hyperkalemia and ECG diagnosis of BrPh and compared their clinical characteristics and outcome with patients with hyperkalemia but no BrPh ECG. Computer simulations investigated the roles of extracellular potassium increase, fibrosis at the right ventricular outflow tract, and epicardial/endocardial gradients in transient outward current. Over a 6-year period, 15 patients presented severe hyperkalemia with BrPh ECG that was transient and disappeared after normalization of their serum potassium. Most patients were admitted because of various severe medical conditions causing hyperkalemia. Six (40%) patients presented malignant arrhythmias and 6 died during admission. Multiple logistic regression analysis revealed that higher serum potassium levels (odds ratio, 15.8; 95% CI, 3.1-79; P=0.001) and male sex (odds ratio, 17; 95% CI, 1.05-286; P=0.045) were risk factors for developing BrPh ECG in patients with severe hyperkalemia. In simulations, hyperkalemia yielded BrPh by promoting delayed and heterogeneous right ventricular outflow tract activation attributed to elevation of resting potential, reduced availability of inward sodium channel conductance, and increased right ventricular outflow tract fibrosis. An elevated transient outward current gradient contributed to, but was not essential for, the BrPh phenotype. Conclusions In patients with severe hyperkalemia, a BrPh ECG is associated with malignant arrhythmias and all-cause mortality secondary to resting potential depolarization, reduced sodium current availability, and fibrosis at the right ventricular outflow tract.
Keywords: Brugada syndrome; Sudden cardiac death; hyperkalemia.
Figures



References
-
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. - PubMed
-
- Antzelevitch C; Heart Rhythm Society; European Heart Rhythm Association . Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. - PubMed
-
- Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang C, Huikuri H. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10:1932–1963. - PubMed
-
- Antzelevitch C, Yan GX, Ackerman MJ, Borggrefe M, Corrado D, Guo J, Gussak I, Hasdemir C, Horie M, Huikuri H, Ma C, Morita H, Nam GB, Sacher F, Shimizu W, Viskin S, Wilde AAM. J‐Wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Europace. 2017;19:665–694. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

