[Baloxavir marboxil: a potent cap-dependent endonuclease inhibitor of influenza viruses]
- PMID: 30676002
- PMCID: PMC6372968
[Baloxavir marboxil: a potent cap-dependent endonuclease inhibitor of influenza viruses]
Abstract
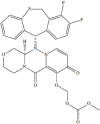
Baloxavir marboxil (5-hydroxy-4-pyridone-3-carboxyl acid) is a new antiviral drug with special efficacy on influenza viruses that acts by inhibiting the cap-dependent endonuclease required for its replication. It is the first representative of the so-called inhibitors of influenza-like PB2. It has shown efficacy against influenza viruses A and B and most strains of animal origin (avian flu). Clinical trials conducted in healthy patients between 12 and 64 years without pathologies and not hospitalized (mild flu) have shown a reduction in the duration of symptoms similar to that obtained by oseltamivir. However, baloxavir is a much more potent inhibitor of viral replication than this drug. It has been shown as a safe and well tolerated drug. A single dose of 40-80 mg is administered the first 48 hours after onset of symptoms. In these trials, strains with moderate sensitivity (PA / I38T mutants) were detected in 2.2% of influenza A (H1N1) pdm09 and in 9.7% of influenza A (H3N2). Although these data could be a good drug to treat mild or moderate influenza, requiring trials in severe influenza and patients with chronic diseases to establish their true clinical utility.
Baloxavir marboxil (ácido 5-hidroxi-4-piridona-3-carboxilo) es un nuevo fármaco antiviral con especial eficacia sobre los virus gripales que actúa inhibiendo la endonuclease cap-dependiente que precisan para su replicación. Es el primer representante de los denominados inhibidores de la proteína básica 2 (PB2) gripal. Ha mostrado eficacia frente a los virus gripales A y B y la mayoría de cepas de origen animal (gripe aviar). Los ensayos clínicos realizados en pacientes sanos de entre 12 y 64 años sin patologías y no hospitalizados (gripe leve) han mostrado una reducción de la duración de la sintomatología parecida a la obtenida por oseltamivir. Sin embargo baloxavir es un inhibidor de la replicación viral mucho más potente que este fármaco. Se ha mostrado como un fármaco seguro y bien tolerado. Se administra una sola dosis de 40-80 mg las primeras 48 horas del inicio de los síntomas. En estos ensayos se han detectado cepas con sensibilidad moderada (mutantes PA/I38T) en el 2,2% de la gripe A (H1N1)pdm09 y en el 9,7% de la gripe A (H3N2). A pesar de estos datos podría ser un buen fármaco para tratar la gripe leve o moderada, precisando de ensayos en gripe grave y pacientes con patologías crónicas para establecer su verdadera utilidad clínica.
©The Author 2019. Published by Sociedad Española de Quimioterapia. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)(https://creativecommons.org/licenses/by-nc/4.0/).
Figures
References
-
- Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. En: Knipe DM, Howey PM (ed.). Fields Virology (6th ed). Lippincott Williams & Wilkins; Philadelphia, 2013, p.1186-1244.
-
- Monto AS. The role of antivirals in the control of influenza. Vaccine 2003; 21:1796-1800. PMID: . - PubMed
-
- Fiore AE, Fry A, Shay D, Gubareva L, Breese JS, Uyeki TM, Centers for Disease Control and Prevention Antiviral agents for the treatment and chemoprophylaxis of influenza-recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60_1-24. PMID: . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous