Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance
- PMID: 30676326
- PMCID: PMC6483520
- DOI: 10.1172/jci.insight.125068
Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance
Abstract
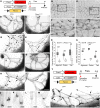
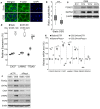
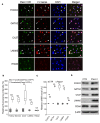
The lymphatic system plays crucial roles in tissue homeostasis, lipid absorption, and immune cell trafficking. Although lymphatic valves ensure unidirectional lymph flows, the flow itself controls lymphatic valve formation. Here, we demonstrate that a mechanically activated ion channel Piezo1 senses oscillating shear stress (OSS) and incorporates the signal into the genetic program controlling lymphatic valve development and maintenance. Time-controlled deletion of Piezo1 using a pan-endothelial Cre driver (Cdh5[PAC]-CreERT2) or lymphatic-specific Cre driver (Prox1-CreERT2) equally inhibited lymphatic valve formation in newborn mice. Furthermore, Piezo1 deletion in adult lymphatics caused substantial lymphatic valve degeneration. Piezo1 knockdown in cultured lymphatic endothelial cells (LECs) largely abrogated the OSS-induced upregulation of the lymphatic valve signature genes. Conversely, ectopic Piezo1 overexpression upregulated the lymphatic valve genes in the absence of OSS. Remarkably, activation of Piezo1 using chemical agonist Yoda1 not only accelerated lymphatic valve formation in animals, but also triggered upregulation of some lymphatic valve genes in cultured LECs without exposure to OSS. In summary, our studies together demonstrate that Piezo1 is the force sensor in the mechanotransduction pathway controlling lymphatic valve development and maintenance, and Piezo1 activation is a potentially novel therapeutic strategy for congenital and surgery-associated lymphedema.
Keywords: Lymph; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

