Engineering the Substrate Specificity of a Modular Polyketide Synthase for Installation of Consecutive Non-Natural Extender Units
- PMID: 30676722
- PMCID: PMC6556384
- DOI: 10.1021/jacs.8b10521
Engineering the Substrate Specificity of a Modular Polyketide Synthase for Installation of Consecutive Non-Natural Extender Units
Abstract
There is significant interest in diversifying the structures of polyketides to create new analogues of these bioactive molecules. This has traditionally been done by focusing on engineering the acyltransferase (AT) domains of polyketide synthases (PKSs) responsible for the incorporation of malonyl-CoA extender units. Non-natural extender units have been utilized by engineered PKSs previously; however, most of the work to date has been accomplished with ATs that are either naturally promiscuous and/or located in terminal modules lacking downstream bottlenecks. These limitations have prevented the engineering of ATs with low native promiscuity and the study of any potential gatekeeping effects by domains downstream of an engineered AT. In an effort to address this gap in PKS engineering knowledge, the substrate preferences of the final two modules of the pikromycin PKS were compared for several non-natural extender units and through active site mutagenesis. This led to engineering of the methylmalonyl-CoA specificity of both modules and inversion of their selectivity to prefer consecutive non-natural derivatives. Analysis of the product distributions of these bimodular reactions revealed unexpected metabolites resulting from gatekeeping by the downstream ketoreductase and ketosynthase domains. Despite these new bottlenecks, AT engineering provided the first full-length polyketide products incorporating two non-natural extender units. Together, this combination of tandem AT engineering and the identification of previously poorly characterized bottlenecks provides a platform for future advancements in the field.
Figures



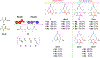
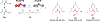
References
-
- Mo S; Kim DH; Lee JH; Park JW; Basnet DB; Ban YH; Yoo YJ; Chen SW; Park SR; Choi EA; Kim E; Jin YY; Lee SK; Park JY; Liu Y; Lee MO; Lee KS; Kim SJ; Kim D; Park BC; Lee SG; Kwon HJ; Suh JW; Moore BS; Lim SK; Yoon YJ, Biosynthesis of the allylmalonyl-CoA extender unit for the FK506 polyketide synthase proceeds through a dedicated polyketide synthase and facilitates the mutasynthesis of analogues. J. Am. Chem. Soc 2011, 133 (4), 976–85. - PMC - PubMed
-
- Chang C; Huang R; Yan Y; Ma H; Dai Z; Zhang B; Deng Z; Liu W; Qu X, Uncovering the formation and selection of benzylmalonyl-CoA from the biosynthesis of splenocin and enterocin reveals a versatile way to introduce amino acids into polyketide carbon scaffolds. J. Am. Chem. Soc 2015, 137 (12), 4183–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

