Surveillance of immunity acquired from poliovirus immunization including vaccination with the Sabin strain-derived inactivated vaccine
- PMID: 30676843
- PMCID: PMC6605838
- DOI: 10.1080/21645515.2019.1572408
Surveillance of immunity acquired from poliovirus immunization including vaccination with the Sabin strain-derived inactivated vaccine
Abstract
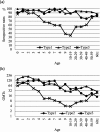
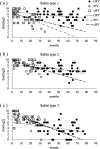
In Japan, routine immunization for polio using the oral polio vaccine (OPV) was suspended in September 2012; subsequently, an immunization program with inactivated polio vaccines (IPVs), the conventional IPV (cIPV) derived from virulent strains, and IPV derived from Sabin strains (sIPV), was introduced. However, the immunity induced by sIPV is not well characterized. This study assessed and compared neutralizing antibodies produced against poliovirus in cases who received doses of OPV or IPV. Serum samples (n = 1186) were collected yearly between 2013 and 2016 as part of the National Epidemiological Surveillance of Vaccine-Preventable Disease. The neutralizing antibody titers for Sabin strain types 1, 2, and 3 in 224 children, aged between 0 and 90 months, were assessed. Seropositive rates after vaccination with OPV or IPV were more than 90%. Neutralizing antibody titers for Sabin type 1 after vaccination with IPV were lower than those with OPV, while those for Sabin types 2 and 3 after vaccination with IPV were significantly higher than those with OPV. Analyses of antibody titer dynamics revealed that the decay of antibody titers for Sabin types 1, 2, and 3 in cases vaccinated with IPV was steeper than those with OPV. Thus, our study showed that although IPV induced a sufficient level of neutralizing antibody, the immunity induced by IPV was not maintained as long as that by OPV. Our study suggested that a long-term survey should be conducted for polio vaccination using IPV and that it might be necessary to consider booster vaccination for IPVs.
Keywords: Poliovirus; Sabin strains; inactivated vaccine; neutralizing antibody; seropositive rates.
Figures
Similar articles
-
Phase II and III Clinical Studies of Diphtheria-Tetanus-Acellular Pertussis Vaccine Containing Inactivated Polio Vaccine Derived from Sabin Strains (DTaP-sIPV).J Infect Dis. 2013 Jul 15;208(2):275-83. doi: 10.1093/infdis/jit155. Epub 2013 Apr 8. J Infect Dis. 2013. PMID: 23568174 Clinical Trial.
-
Immunity status of adults and children against poliomyelitis virus type 1 strains CHAT and Sabin (LSc-2ab) in Germany.BMC Infect Dis. 2010 Dec 9;10:347. doi: 10.1186/1471-2334-10-347. BMC Infect Dis. 2010. PMID: 21143885 Free PMC article.
-
Polio vaccination coverage and seroprevalence of poliovirus antibodies after the introduction of inactivated poliovirus vaccines for routine immunization in Japan.Vaccine. 2019 Mar 28;37(14):1964-1971. doi: 10.1016/j.vaccine.2019.02.034. Epub 2019 Mar 1. Vaccine. 2019. PMID: 30827736
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. Cochrane Database Syst Rev. 2019. PMID: 31801180 Free PMC article.
-
Poliovirus vaccines. Progress toward global poliomyelitis eradication and changing routine immunization recommendations in the United States.Pediatr Clin North Am. 2000 Apr;47(2):287-308. doi: 10.1016/s0031-3955(05)70208-x. Pediatr Clin North Am. 2000. PMID: 10761505 Review.
Cited by
-
Immunogenicity and Safety of Inactivated Sabin-Strain Polio Vaccine "PoliovacSin": Clinical Trials Phase I and II.Vaccines (Basel). 2021 May 29;9(6):565. doi: 10.3390/vaccines9060565. Vaccines (Basel). 2021. PMID: 34072466 Free PMC article.
-
Immune persistence of an inactivated poliovirus vaccine derived from the Sabin strain: a 10-year follow-up of a phase 3 study.EClinicalMedicine. 2023 Sep 14;64:102151. doi: 10.1016/j.eclinm.2023.102151. eCollection 2023 Oct. EClinicalMedicine. 2023. PMID: 37745024 Free PMC article.
-
Evaluation of antigenic differences between wild and Sabin vaccine strains of poliovirus using the pseudovirus neutralization test.Sci Rep. 2019 Aug 19;9(1):11970. doi: 10.1038/s41598-019-48534-1. Sci Rep. 2019. PMID: 31427704 Free PMC article.
-
Long-Term Persistence of Anti-Poliovirus Antibody Titers After Two-Dose Booster Immunization with Conventional Inactivated Poliovirus Vaccine Among Japanese Adults: 10-Year Observational Study.Vaccines (Basel). 2025 Apr 23;13(5):447. doi: 10.3390/vaccines13050447. Vaccines (Basel). 2025. PMID: 40432059 Free PMC article.
-
The progress of postapproval clinical studies on Sabin IPV.Hum Vaccin Immunother. 2022 Dec 31;18(1):1-4. doi: 10.1080/21645515.2021.1940653. Epub 2021 Jul 2. Hum Vaccin Immunother. 2022. PMID: 34213408 Free PMC article.
References
-
- World Health Organization Global eradication of poliomyelitis by the year 2000. Forty first World Health Assembly, resolution 41.28. WHO, Geneva, Switzerland, 1988.
-
- Polio Grobal Eradication Initiative Polio eradication & endgame strategic plan 2013–2018. WHO, Geneva, Switzerland, 2013.
-
- WHO Polio vaccines: WHO position paper – March, 2016. Wkly Epidemiol Rec. 2016;91(12):145–68. PMID:27039410. - PubMed
-
- Murdin AD, Barreto L, Plotkin S. Inactivated poliovirus vaccine: past and present experience. Vaccine. 1996;14(8):735–46. PubMed PMID: 8817819. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
