Serotonin and neuropeptides are both released by the HSN command neuron to initiate Caenorhabditis elegans egg laying
- PMID: 30677018
- PMCID: PMC6363226
- DOI: 10.1371/journal.pgen.1007896
Serotonin and neuropeptides are both released by the HSN command neuron to initiate Caenorhabditis elegans egg laying
Abstract
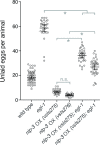
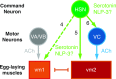
Neurons typically release both a small-molecule neurotransmitter and one or more neuropeptides, but how these two types of signal from the same neuron might act together remains largely obscure. For example, serotonergic neurons in mammalian brain express the neuropeptide Substance P, but it is unclear how this co-released neuropeptide might modulate serotonin signaling. We studied this issue in C. elegans, in which all serotonergic neurons express the neuropeptide NLP-3. The serotonergic Hermaphrodite Specific Neurons (HSNs) are command motor neurons within the egg-laying circuit which have been shown to release serotonin to initiate egg-laying behavior. We found that egg-laying defects in animals lacking serotonin were far milder than in animals lacking HSNs, suggesting that HSNs must release other signal(s) in addition to serotonin to stimulate egg laying. While null mutants for nlp-3 had only mild egg-laying defects, animals lacking both serotonin and NLP-3 had severe defects, similar to those of animals lacking HSNs. Optogenetic activation of HSNs induced egg laying in wild-type animals, and in mutant animals lacking either serotonin or NLP-3, but failed to induce egg laying in animals lacking both. We recorded calcium activity in the egg-laying muscles of animals lacking either serotonin, NLP-3, or both. The single mutants, and to a greater extent the double mutant, showed muscle activity that was uncoordinated and unable to expel eggs. Specifically, the vm2 muscles cells, which are direct postsynaptic targets of the HSN, failed to contract simultaneously with other egg-laying muscle cells. Our results show that the HSN neurons use serotonin and the neuropeptide NLP-3 as partially redundant co-transmitters that together stimulate and coordinate activity of the target cells onto which they are released.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017:6 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5302148/ - PMC - PubMed
-
- Baker KG, Halliday GM, Hornung J-P, Geffen LB, Cotton RGH, Törk I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience. 1991;42: 757–775. - PubMed
-
- Appel NM, Wessendorf MW, Elde RP. Thyrotropin-releasing hormone in spinal cord: coexistence with serotonin and with substance P in fibers and terminals apposing identified preganglionic sympathetic neurons. Brain Res. 1987;415: 137–143. - PubMed
-
- Henry JN, Manaker S. Colocalization of substance P or enkephalin in serotonergic neuronal afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1998;391: 491–505. - PubMed
-
- Hökfelt T, Pernow B, Wahren J. Substance P: a pioneer amongst neuropeptides. J Intern Med. 2001;249: 27–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

