Nimesulide-induced hepatotoxicity: A systematic review and meta-analysis
- PMID: 30677025
- PMCID: PMC6345488
- DOI: 10.1371/journal.pone.0209264
Nimesulide-induced hepatotoxicity: A systematic review and meta-analysis
Abstract
Objective: This study aimed to evaluate the risk for hepatotoxicity with nimesulide, a non-steroidal anti-inflammatory drug (NSAID) available in Republic of Korea but withdrawn from the market in several countries.
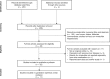
Methods: A systematic review and meta-analysis were conducted of studies retrieved from PubMed, EMBASE, Cochrane, the Research Information Sharing Service and ClinicalTrials.gov up to September 2017. All studies reporting nimesulide-associated hepatotoxicity in patients as compared with the unexposed or the exposed to other NSAIDs were included. Studies using spontaneous reporting databases were included to estimate reporting odds ratio (ROR) of hepatotoxicity associated with nimesulide exposure. The association between nimesulide use and hepatotoxicity was estimated using relative risk (RR) and ROR with 95% confidence interval (CI).
Results: A total of 25 observational studies were eligible for review. In a meta-analysis of five observational studies, nimesulide was significantly associated with hepatotoxicity [RR 2.21, 95% CI 1.72-2.83]. From studies using spontaneous reporting databases (n = 6), rates of reported hepatotoxicity were significantly higher in patients using nimesulide, compared with those treated with other NSAIDs [pooled ROR 3.99, 95% CI 2.86-5.57]. Of a total of 33 patients from case studies and series, the majority (n = 28, 84.8%) were female, and the mean age (± standard deviation) was 56.8 (± 15.6) years. Almost half of the patients on nimesulide (45.5%) either required liver transplantation or died due to fulminant hepatic failure, of whom a third developed hepatotoxicity within less than 15 days of nimesulide administration.
Conclusions: Our study findings support previous reports of an increased risk for hepatotoxicity with nimesulide use and add to existing literature by providing risk estimates for nimesulide-associated hepatotoxicity. As the limited number of studies with primarily observational study designs were included in the analysis, more studies are needed to further describe the effects of dose and length of treatment on the risk for hepatotoxicity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy.Br J Clin Pharmacol. 2016 Jul;82(1):238-48. doi: 10.1111/bcp.12938. Epub 2016 Apr 27. Br J Clin Pharmacol. 2016. PMID: 26991794 Free PMC article.
-
Serious liver injury induced by Nimesulide: an international collaborative study.Arch Toxicol. 2021 Apr;95(4):1475-1487. doi: 10.1007/s00204-021-03000-8. Epub 2021 Mar 24. Arch Toxicol. 2021. PMID: 33759010
-
Fatal hepatoxicity secondary to nimesulide.Eur J Clin Pharmacol. 2001 Jul;57(4):321-6. doi: 10.1007/s002280100312. Eur J Clin Pharmacol. 2001. PMID: 11549211
-
Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage?World J Gastroenterol. 2010 Dec 7;16(45):5651-61. doi: 10.3748/wjg.v16.i45.5651. World J Gastroenterol. 2010. PMID: 21128314 Free PMC article. Review.
-
Systematic review: ibuprofen-induced liver injury.Aliment Pharmacol Ther. 2020 Mar;51(6):603-611. doi: 10.1111/apt.15645. Epub 2020 Jan 27. Aliment Pharmacol Ther. 2020. PMID: 31984540
Cited by
-
Excessive Self-Medication with Prescription NSAIDs: A Cross-Sectional Study in Kosovo.Pharmacy (Basel). 2024 Jun 12;12(3):93. doi: 10.3390/pharmacy12030093. Pharmacy (Basel). 2024. PMID: 38921969 Free PMC article.
-
Awareness of the use of non-steroidal anti-inflammatory drugs: A cross-sectional study.Med Int (Lond). 2025 Jun 16;5(5):49. doi: 10.3892/mi.2025.248. eCollection 2025 Sep-Oct. Med Int (Lond). 2025. PMID: 40599153 Free PMC article.
-
Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993-Mid 2020: A Comprehensive Analysis.Medicines (Basel). 2020 Sep 29;7(10):62. doi: 10.3390/medicines7100062. Medicines (Basel). 2020. PMID: 33003400 Free PMC article. Review.
-
Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective.Biochem Pharmacol. 2020 Oct;180:114147. doi: 10.1016/j.bcp.2020.114147. Epub 2020 Jul 10. Biochem Pharmacol. 2020. PMID: 32653589 Free PMC article. Review.
-
Repurposing Nimesulide, a Potent Inhibitor of the B0AT1 Subunit of the SARS-CoV-2 Receptor, as a Therapeutic Adjuvant of COVID-19.SLAS Discov. 2020 Dec;25(10):1171-1173. doi: 10.1177/2472555220934421. Epub 2020 Jun 5. SLAS Discov. 2020. PMID: 32500793 Free PMC article.
References
-
- Chatterjee S, Pal J, Biswas N. Nimesulide-induced hepatitis and toxic epidermal necrolysis. Journal of postgraduate medicine. 2008;54(2):150–1. Epub 2008/05/16. . - PubMed
-
- Wober W. Comparative efficacy and safety of nimesulide and diclofenac in patients with acute shoulder, and a meta-analysis of controlled studies with nimesulide. Rheumatology (Oxford, England). 1999;38(suppl_1):33–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

